  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ABOUT CONTACT CONTRIBUTION OVERVIEW TUTORIALS LEGAL/COPYRIGHT A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Dates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CREATED 9/19/2012 WARNING:
This site deals only with the corporate corruption of science, and makes no inference about the motives or activities of individuals involved.
|
OPINION ONLY
George Louis Carlo (Part 2) (Tobacco & Other 1989-93) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Around 1991, Laura Carlo, a reporter on NPR public radio was doing pieces on passive smoking. Was she related ? |
Some key documents
• Return to Part 1 — Early years; Dow Chemicals, Agent Orange, etc.
working for the tobacco industry through their jointly-owned company,
Health & Environmental Sciences, Inc.
The threat of passive-smoke (ETS)
The industry's knowledge about the imminent release of the Environmental Protection Agency (EPA) risk characterization of second-hand smoke as a "known human carcinogen" led to them pouring millions of dollars into anti-EPA propaganda. Philip Morris led the way, with the Tobacco Institute and RJ Reynolds following behind.
Carlo and LeVois became embroiled in a number of these projects:
- The planning of the November 1989 McGill University ETS Conference by Andrew Whist's group at PM International, Corporate Affairs division. This was to be a closed conference with a pre-determined output — mainly a 'proceedings' booklet — which would be widely circulate and exonerate passive-smoking. It would also attack the EPA for its lack of "sound-science."
- The development via PR and the media of two general 'themes' which could be attached by repetition to anti-smoking science as labels:
- "junk-science" was the slogan promoted through "The Advancement of Sound Science Coalition (TASSC) and the Manhattan Institute.
- "scientific bias" was promoted through a loaded form of push polling, which pretended to be research. This was designed to prove that anti-tobacco science couldn't be trusted to be impartial.
- The Tobacco Institute also had its "Scientific Witness Teams" (SWT) and their "Truth Squads" which were sent off to various states to act as witnesses against threats of State, regional or local public smoking bans. Recruiting and training scientists as mouthpieces, without the risk of a rogue recruit becoming a whistleblower, needed a careful selection process. It was carried out at arm's length through lawyers and some independent recruiters and trainers.
- RJ Reynolds had been developing its tube cigarette ( Premier and later Eclipse) which delivered nicotine vapour by heating rather than burning the tobacco, and without the visible smoke and (supposedly) carcinogenic 'tar' compounds of tobacco. They also needed a stable of credible 'independent' scientists who would be paid to attend conferences; write articles, op-eds and letters-to-the editor; to promote the new way of smoking.
1989 May 31: Tobacco Institute document: "Status of Identification, Recruitment and Training of Academic Scientists for ETS-related Matters." This had been prepared for the May 31 Communications committeee meeting.
[The Communications committeee consisted of the PR operatives and lobbyists for five of the cigarette companies]
This document (which was developed primarily as a speech) outlines the elaborate procedures that they followed when recruiting corrupt or corruptable academic scientists.
1. Review of Assignment and Authority from April 7 Executive Commitee meeting.It was important that these 'witnesses' maintain distance from the industry, to the point where they could deny being employed by tobacco companies.
- 12 - 15 Academic Scientists on ETS to publish in the scientific literature, testify before Congress and major regulatory agencies. Participate in scientific conferences.
- Aircraft ventilation expert to buttress cabin air quality activity
[The airline cabin crews were lobbying for smoking bans on domestic flights.]- Budget authority — $1.05 million for the balance of 1989
2. Institute staff is handling overall coordination.The procedures used for recruitment were elaborate to prevent any infiltration of their scientific flanks. The candidates were paid for two weeks to review literature on passive smoking, and then selected on the basis of whether their reports were favourable to the industry's position. It they were, they were then approached by lawyers before being handed over to George Carlo and corporate PR experts for media training.3. Progress to date ETS Scientists.
- Clausen Ely of Covington & Burling [tobacco lawyers] is coordining day-to-day identification and recruitement activity.
- Efforts to identify began last fall.
- Covington [& Burling] retained an expert witness search firm, Weinberg & Associates.
- Myron Weinberg developed a list of 17 candidates
- Recruitment procedures [See actual document]
Five had been dropped; five are in pipeline reviewing literature. Those finally selected were also to be trained to appear on TV, radio and at press conferences to deny that there were any causal links between passive smoking and adverse health by questioning the reliability of the science.
- Since mid-April Weinberg and Ely have reviewed the resumes and published paper of the 17 candidates identified last fall and have made initial contact with about half of them;
It goes on to discuss their problems with the recruitment of an "aircraft ventilation expert" and how Gray Robertson (of HBI) stepped in to fill the gap — and later the help they received in finding a mouthpiece with legitimate credentials.
- In the next week or so, Ely, Weinberg and a new recruiter/trainer, George Carlo, should complete conference calls with these five candidates to ascertain their views on the literature.
- In Addition, Tom Borelli of PM [top Philip Morris misinformation specialist] suggested two candidates from the faculty of the New York Medical College ([Prof. Joseph M] Wu and [Lawrence M] Wexler).
- Ely has contacted both. Both have reviewed the ETS literature. Conference calls indicate that their views of the literature are consistent with the industry's.
- Ely and Carlo are scheduled to meet them (Wu and Wexler) on June 23rd at Philip Morris' office in Manhattan.
- Ely and Carlo also hope to meet with the other NYC-area prospects (Godfrey and Gutterplan) on the 22nd.
- Meanwhile, Weinberg has developed a second list of 12 candidates and their published papers for our review. And the process will continue until we reach our goal.


1989 Jun 15: Sam Chilcote, the President of the Tobacco Institute, used a later version of the above document as his speech notes to the tobacco company CEOs who constituted the "Executive committeee". He has added a few handnotes.
[Worth checking both] 

1989 June 16: /E Philip Morris completely restructured their fight-back operations. They merged the Corporate Affairs and Science & Technology divisions to create Corporate Scientific Affairs under top lawyer Steve Parrish, and they shifted many of their key executives around.
This restructuring also resulted in the employment of Newman Partnership Ltd, to "cut-out" direct contact with scientists offering to do dubious research, and also the establishment of an internal ETS Management committeee (EMC) which would supervise all fake science and misinformation activities. The members of this committee included both Tom Borelli and Fred Newman, and it produced this report on how they should handle the growing problems of second-hand smoke (ETS).
George Carlo clearly had access to this primary ETS planning document also since, in his proposals, he was able to echo their own requirements.
Draft ETS plan  Notes on changed responsibilities
Notes on changed responsibilities 

1989 June 16: The Newman Partnership Ltd. (NPL) was run by 'Larry' [Lloyd] and Fred Newman and their wives as a PR/scientific communications firm.
[Fred Newman was the inhouse legal counsel for Philip Morris, and it is not clear whether Philip Morris knew he had a share of the company with his brother Lloyd].
From mid 1989, NPL had a contract with Philip Morris to run a program of "scientific communications" in support of the Science & Technology (S&T) division of Corporate Scientific Affairs (run by Tom Borelli). S&T handled scientific issues [The production of misinformation and countering of science.] and NPL provided a cut-out service designed to keep the guns-for-hire operators at arm's length in case anyone ran into legal problems. 

1989 Aug 5: In parallel to the changes at Philip Morris, the Tobacco Institute has contracted Larry Holcomb who ran the science-for-sale operation Holcomb Environmental Services, to reorganise their Scientific Witness Team (SWT) of supposedly 'independent' scientists, to make them more effective.
Holcomb came up with the strategy of allocating 'subcategories' of potential problems to pairs of scientists (one to act as a backup). They would be required to publish and speak on these subjects, and thus create a perception of expertise, and would then be available to respond rapidly to any adverse health claims appearing in the scientific literature or popular press.
This document says that in their sub-categories, they are paid to provide the following services:
The will then be available to persuade other scientists via letters to the editor of scientific journals or presentations/discussions, etc. and also act as trainers for the other industry scientists.
- Review of past and present literature.
- Fast review of new data.
- Fast access to new information.
- Attendance at science sessions on the subject.
- Taking part in the science and/or scientific societies treating these subjects.
- Coordinating and sharing information with other scientists identified in their subcategory.
Holcomb lists the Tobacco Institute's current Scientific Witness Team (all well known tobacco scientist) with suggestions as to their potential specialist subcategories:
Maurice LeVois and George Carlo:[Re: Cervical Cancer. The March 1989 Journal of the American Medical Association (JAMA) had carried an article by Martha Slattery which concluded that cervical cancer was linked to ETS. It was a weak-association, possibly made on dubious grounds, and she came under attack from Carlo's friend, Dr Ernst Wynder, of the American Health Foundation.]
- Cervical Cancer / Cancers other than lung cancer.
- Epidemiology: Methodology and Statistics
1989 Aug 9: /E A later update EMC document, reported, under the heading of
Public Relations
Identified the Newman Partnership, a public relations firm specializing in scientific issues/controversies, to execute the ETS program. The group focuses on scientists as well as the science media to influence the views of the general media.


Also a more explicit version was prepared (probably for management) which says
Identification of a science communications consultant, the Newman Partnership, which would undertake a scientists' lobbying program to get scientists to reevaluate their views on ETS.


[This is a clear admission that the Newman Partnership was contacted to develop pseudo-science, PR and lobbying: and not involved in real science.]
1989 Aug 10: Maurice LeVois as "President" writes to disinformation executive Tom Borelli at Philip Morris. He is writing from San Francisco on Health & Environmental Science Corporation letterhead on behalf of himself and Carlo.
Dear Dr Borelli [... they are not yet on firstname terms]They want $7,5000 in advance just to write up a formal scientific protocol. This would be needed to make the whole project look as if it were genuine scientific research if any documents leaked.
I would like to follow up our recent discussion about ways to test the hypothesis that scientists are systematically misinformed about ETS. What follows is a brief recap of the basic concepts we discussed, and a request for approval to develop a research protocol to test these ideas.
I think that there is ample reason to believe that the information, attitudes, and beliefs of scientists are not entirely informed by an objective reading of the scientific literature with regard to ETS. Part of this problem stems from a tendency (not unique to scientists) for scientists to read only information that is consistent with their preexisting views, and to disregard information that challenges their views.
[He now jumps from his pretense at scientific inquiry, to scientific lobbying and public relations without taking a breath.]
Changing the way scientists select and review the ETS literature will be difficult, and success will depend upon our ability to develop a properly focused approach to communicating with them.
[So the idea is to 'change' scientists' views, not discover their bias.]
One useful step towards the goal of fostering greater scientific objectivity in the ETS debate [a common euphemism in the industry for promoting a pro-smoking viewpoint] that we discussed earlier is to obtain objective evidence that an information bias exists. This evidence alone could encourage some scientists to reevaluate their ETS position. Scientifically rigorous survey research could provide such evidence.
['Scientifically rigorous' depends here on the rigor of the scientist — and the whole purpose of hiring Carlo and LeVois is to ensure that someone claiming to be 'independent' collected it — not the tobacco companies.]
I. Information Survey
To test the hypothesis about systematic ETS misinformation, I propose that we develop a questionnaire and conduct a survey that presents information to a sample of scientists in the form a specific questions about ETS. Topics that the National Research Council has identified as being poorly understood, or lacking adequate data, could serve as the primary topic pool for the survey.
['topic pool' refers to other pollutants being compared - such as dioxins, radon, Alar, etc]
If a significant number of scientists respond as if there were good data to support conclusions [that ETS and the other topic pool substances are harmful] then this should be interpreted as objective evidence of bias."
[So by carefully selecting disputable topics for the survey, they would guarantee to find evidence of so-called "bias" against a topic that almost no one disputed as potentially dangerous.]
II Focus Group Research
Since the survey discussed above is intended to provide objective evidence of an information bias we already have reason to believe exists, a parallel effort could proceed to identify an effective intervention to overcome this bias.
[In parallel with the so-called rigorous science — they would run a series of misinformation campaigns targeting selected groups indentified by their focus groups.]
As you, know, George Carlo and I would like to conduct research for PM along the lines outlined above. We propose developing a detailed research protocol for the survey portion of this work, and I believe that it would be wise to work closely with PM at this stage in order to focus our research on areas of greatest value to the client.
George has already mentioned that we would request a $7500 advance payment that would be billed against by us during the start up phase of this project.
In fact, the outline of the project is little more than a PR program to promote the idea that any scientist with any adverse view of the safety of tobacco smoke is naturally biased — and therefore can't be trusted to do research.


[Both Carlo and LeVois published independent bias papers in 1992 — but by then they were no longer working together.]
| The EPA's Risk Assessment |
|---|
| At this time the Environmental Protection Authority was working on an assessment of the potential health risks for passive smoking (ETS). They had already released some information suggesting that up to 3000 people a year died prematurely (mainly from increased rates of lung-cancer) because they were subject to high levels second-hand smoke.
Since tobacco smoke itself was cancer-producing, they had decided to also classify ETS as a Class A carcinogen (known human cancer producer) unless the science could prove otherwise. [Note that the Class doesn't take into account the dosage or extent of exposure, just the danger inherent in the toxic substance itself.]. |
1989 Aug 11: Larry Holcomb's Scientific Witness Team (Aug 5) proposal to the Tobacco Institute must have been acted upon almost immediately. Only a few days later this memo circulated within the Tobacco Institute.
The Institute currently has a team of nine scientific consultants (the so-called "second team" ) who provide expert testimony before state and local legislative and regulatory bodies, attend and report on scientific meetings, and prepare "quick and dirty" critiques of scientific reports and letters-to-editors of general and scientific publications. The nine are:Larry Holcomb coordinates the activities and "continuing education" of this group. Carlo and LaVois, relative newcomers to the team, have not yet been used as witnesses but will be available for next year's legislative season. Peterson and Weeks, famous for their "Truth Squad" media tours, now only rarely appear as witnesses for TI.
- Larry Holcomb
- Joe Pedelty
- Walt Decker
- Barry Seabrook
- Larry Halfen
- Jack Peterson
- David Weeks
- George Carlo
- Maurice LaVois


[The "first team" and "third team" were respectively paid-off university professors, and executives of a couple of Indoor Air Quality (IAQ) testing companies who contracted with the Tobacco Institute to produce doctored results to show that tobacco smoke was only ever a minor cause of poor air quality.
The "Truth Squad" was a small flying-circus operation where a couple of eminent and 'independent' air-quality scientists would suddenly turn up in a city where smoking bans were being proposed and deny the existance of scientific evidence of harm from tobacco smoke.]
1989 Aug 22: Sam Chilcote writes to members of the Tobacco Institute's Executive committeee.
Per your request at our August 17 meeting, I am enclosing a list of scientists who currently consult with the industry on ETS and indoor air quality issues. As counsel advised, please treat this information with utmost discretion.There are a few variations of this list in the files of different companies. The lists are divided into:
We now have a total of 37 consultant scientists. Of these, 14 are affiliated with or are faculty members at academic institutions. The remaining scientists, each of whom has solid credentials, consult on a full-time basis.
- Academics ("first team")
- Full time ETS Consultants ("second team")
- Full-time consultants on IAQ ("third team")




[This also became the primary list used by Tom Borelli (below) for selecting many speakers the McGill University ETS Conference in November 1989]
1989 Aug 30: Tom Osdene, the Vice President at the head of the Philip Morris Research Center in Richmond, has received a complete list of tobacco industry consultants from the Tobacco Institute. He travels extensively in Europe and is an experienced recruiter of "scientific friends".
[In later court cases he regularly pleaded the Fifth Amendment about his recruitment activities.]
He appears here to be checking US potential contributers to the McGill University ETS Conference to ensure that he haa copies of their documentation.
Osdene uses the headings "BUS" (Business?); "BIB" (Bibliography) and "BIO" (Biography) and also corrects spelling and initials. He is also preparing computerised WHTCOATS.TXT (WhiteCoats text) which was completed on September 5 1989 and another file labled COATS.TXT the day before.
| Whitecoats were then a new type of recruited 'sleeper' scientist/medico who had no traceable connections to any tobacco company and no history of having received tobacco grants. They operated through the cut-out services of lawyers Covington & Burling, and were being recruited in the UK, and throughout Europe, Scandinavia, and Asia at this time.
Since they had no actual knowledge of tobacco industry problems or of the science, the McGill University conference was seen as a training ground for them. By reading prepared speaches, they could also get on the record in their home countries (via the published and translated conference proceedings) as having been an 'invited expert keynote speaker' at an international smoking and health conference. |
These are clearly checklists of personal documents received for scientist invited to the McGill Conference in Canada. While the conference was run by Philip Morris International under the control of Andrew Whist, head of PMI Corporate Affairs, Thomas Osdene was obviously overseeing the selection of US domesic scientific lackies.
Carlo and LaVois are listed as "Full-time Consultants on ETS" , but LeVois has provided no documentation (he is in San Francisco, and probably wasn't invited). It is pretty safe to assume that this is a list of well-known safe scientists who can be used in this 'closed' (by invitation only) conference.
[ Joseph Wu of the New York Medical College, who had only been recruited and trained by Carlo a few months before, was, in fact, credited in the proceedings as having initiated this conference and goven the prestigious position of Chairman.]


With the exception of "D.Johnson" (unknown) everyone listed here is a known long-term tobacco scientist or science-for-sale entrepreneur. They are all revealed in the tobacco archive documents to have lobbied extensively for Philip Morris in the USA.
See also the Results of this conference.



Health and Environmental Sciences Group. Ltd (HESG) Washington DC.
1989 Sep 13: Newman Partnership Ltd (NPL) sends a draft plan for discussion to the EMC committeee of Philip Morris, It identifies its own role as "scientific public relations counsel" and sets a monthly fee of $117,000 for a sixteen month period.
They have employed the notorious John Scanlon as Senior Counsel and Management Supervisor. 

1989 Oct 6: The Newman Partnership has had a meeting with Philip Morris's EMC committeee about
Science Communications - "Launch" Projects.They also had plans to roll our a series of science-writers workshops on "poor science" (later called "junk-science") which were to be initially held at Harvard or Georgetown University with Kraft General Foods (a PM subsidiary) as the overt sponsor.
Based on our meeting today, we have redefined our Science Communication projects
- Challenge study : NAS study for Surgeon General's Report to be done by LeVois ($25,000) with the Goal:
[ie.The NAS (National Association of Science) had published a study which supported the EPA's decision that passive smoking was harmful. This was also in agreement with the Surgeon-General's annual smoking report.
- Show cumulative bias over long term
- Determine extent of the willingness to reconsider NAS conclusions
- Attempt to identify possible effective intervention communication to reduce bias.
LeVois was to challenge the NAS Study by finding evidence of bias among the contributors. ]
- Survey "XYZ Substances": to be done by George Carlo ($70,000) with the Goal:
[This is the "Bias Study" of 1000 scientists from 5 different scientific disciplines. It is a phone survey to compare their opinions of ETS with three (XYZ) other substances (dioxin, radon, alar)]
- Expose ETS prejudice
- Show ETS as not a major issue
- Survey : "Minimum standards for research acceptability" to be done by Carlo ($60,000) with the Goal:
[This was the "Health Scientist Survey" which also appears to be a preliminary concept for what later became the London Principles and Good Epidemiological Practices.(GEP)]
- Expose shortcomings in EPA and other studies
- Invalidate EPA methodology
Other projects included the promotion of the notorious "Harvard Professor and heart expert" Carl Seltzer — who made millions from the tobacco industry despite the fact that he was only ever an honorary graduate resesearcher at the Peabody Museum.


1989 Oct 17: Tom Borelli has returned this NPL memo to Larry Newman following their meeting with George Carlo and his assistant Patricia Doseberg. He writes across the top:
"Larry, Please review and correct. I have talked today with Carlo, Coughlin, Cherney and MobyThe NPL has tried to word the plan in scientific protocol terms, but they can't avoid the obvious public relations and lobbying content which is their raison d'étre. At this time they plan to do a series of projects: [Slightly paraphrased]
Clarify the planned science opinion studies and initiate development of the protocols.
[JR Coughlin of Kraft-General Foods was also doing a bias study for coffee makers;
Arlene Cherney and S Moby were on NPL staff.
[Of course most intelligent scientists and doctors will naturally be opposed to smoking — and see it as a costly, addictive, and useless practice to be discouraged. This distorted study protocol is designed to show up such precautionary opinions as evidence of 'scientifically bias'.
- Minimum Criteria of Acceptability for Scientific Studies
The study should reflect opinion, by scientific specialties, of criteria for deciding the appropriate degree/linkage of a relationship between an environmental factor and a disease.
[This project is to be a causation-criterea study designed to create a 'purist' outcome. (Setting up restrictive rules for when a scientist could say "Disease A was caused by B.") ]- Purpose of the study:
[To be of use to the tobacco industry it requires critical press attacking the EPA and NAS statements that smoking 'caused' lung-cancer, etc., and publication of the new rules in reputable scientific/medical journals.]
- Impact on the EPA Risk Assessment Advisory Report.
- Publication in reputable scientific/medical journals so as to establish these criteria in the minds of scientists as being widely accepted and appropriate.
- Publication in general news media
- It will consider weaknesses in the National Academy of Science (NAS) study, the Surgeon General's Report, and influential studies like that of Hirayama,
[The Japanese Hirayama study was very large and convincing. It was used by both the NAS and the Surgeon-General's advisory board as hard evidence that ETS harmed non-smokers.]- Baseline Review of NAS Study on ETS to determine "What It Really Meant" by interviewing members of the original study group, and creating a 'proper interpretation'.
[They obviously thought that it would be possible to convince some of the scientists involved in the NAS study to equivocate, and express 'scientific reservation' about absolute conclusions. This equivocation could then be promoted as 'doubt' or even 'recanting'.]- Study on the Source of Scientist Bias (in two parts)
- What scientists think the NAS study said, and what it "really said."
[This aimed to expoit any equivocation by the NAS study principles, in comparison with the statements of those questioned in the phone survey.]- What influences Scientists in terms of bias.
[What happens when they know the main topic is cigarette smoke?]
- XYZ Study
- Test perceptions of the degree of risk of ETS and of two other substances (dioxin?, Alar?, other?) on the bias of scientific evidence :
[The use of false comparisons (acute vs chronic: universal vs probable) can then be exploited as evidence of bias. The vague life-time risk of a range of smoking-related diseases is being compared to mutagenic and debilitating conditions like dioxin poisoning of children.]
- Without revealing the name of the substances
- And then when the substances are identified
- Purpose: To determine if scientists/physicians display a prejudice against substances when identified versus "blind" analysis based on evidence only).
- Deadline: Prior to the release date of the EPA's ETS Risk Assessment report
In simplistic PR terms, this is a fail-proof strategy, and the industry played it to the hilt. At this time it was almost the only effective weapon that they had against the EPA's classification of passive smoking as a cancer risk to non-smokers.]


1989 Oct 27: Internal Tobacco Institute memo with "a preliminary agenda for the Scientific Witness Team (SWT) meeting, Sessions will take place at the Sheraton-Carlton."
Carlo is to speak on his designated sub-category of Cervical Cancer. 

1989 Oct 31: Larry Newman of The Newman Partnership is at the Society for Risk Assessment Conference in San Francico. He memos Tom Borelli, Nelson Beane and Tom Osdene at Philip Morris.
He is reporting on the paper being presented by EPA's principle ETS investigators, Kenneth Brown (KB) and Douglas Crawford-Brown (DCB), and is clearly out of his depth in scientific terms.
However as a lobbyist and PR man, he is also collecting scuttlebutt — and recording comments that might be useful to the tobacco industry. He has tape recorded both the conference sessions and some private conversations. He describes the two EPA investigators who have been looking into whether cigarette smoke might have some sort of a synergistic reaction with radon gas (present in US granite areas) as does asbestos:
KB is a prototypic "absent minded professor". He dresses in sweater and tie and bumbles through his presentations, thinking aloud as he goes. He is the ideal person to have on the witness stand if you want to "take him apart."They are doing further work on ETS, but are not happy with the results so far.
DCB looks like a prototypic nerd until he opens his mouth. He is brilliant on his feet. I think this man could be "acquired" and should be pursued for that possibility.
In private conversation with me during a break in the workshop, DCB said he has lots of questions about his ETS work. "Something is going on with ETS and radon but I can't say what with great confidence."Larry Newman had suggested to them that they might work through NPL in the future on radon and dioxins, and Kenneth Brown had tentatively agreed.[It was not specified that this was for a tobacco company.]
About his mathematical model on ETS components for EPA he said, "I have no confidence because there are so many (carcinogenic) components. What do I look at — my 10 favorites on Tuesday." The EPA is pushing him [to finish the ETS work] so they can go before the SAB, but he says he won't go because they will "take him apart".
He doesn't believe EPA can use his materials (or intends to) as a basis for regulatory/political action. He promised to send me a draft of his entire work of which ETS/Radon is a part.
At this conference the American Industrial Health Council (the lobby subsidiary of the Chemical Manufacturers Association) was promoting their latest attempt at establishing standards for Good Epidemiological Practices (GEP). [A job later taken over by Philip Morris] Larry Newman reports:
EPA staff reviewed a draft document setting standards for publication (and acceptance?) of research. They were out of copies before I got one, but I called Tom Borelli and George Carlo and gave them the following name and address [of the] American Industrial Health Council.


1989 Nov 3 - 4: McGill University ETS Symposium.
Carlo is not included on the speakers list despite having helped with the recruitment and vetting of many participants. It may well be that he was seen as one of the organisers, rather than as a participant — of that he was doing witness work for the TI's SWT — or was working for some other industry at the time.
This was Philip Morris International's closed-conference of about 80 international tobacco scientists. They were in-house scientist trusted scientific consultants from the US/Canada and Europe, and the newly recruited 'Asian WhiteCoats' attended their first tobacco conference. They were all in Canada at Philip Morris and Tobacco Institute's expense.
Probably another 30-50 staff and organisers also attended and a few from industry-funded think-tanks. It was a massive and expensive undertaking run by Andrew Whist at PMI Corporate Affairs.
The aim of the seminar was to develop a 'consensus' among the commissioned consultants and in-house scientific staff about ETS and exchange views about how the EPA and other agency science could be countered. The 'consensus views' could then be promoted as those of a genuine conference, and proof of doubt in the scientific community that passive smoking was a health problem.
This 'proof' was was accumulated in the vetted conference speeches, which were then improved, selected and collated into the conference proceedings which were to be publish and distribute as a large booklet by the Institute for International Health & Development (IIHD) run by Paul Dietrich.
The booklet was translated into multiple languages and circulated around the world to be used as a university textbook in many countries. [See other material on McGill]


[The IIHD was actually a Philip Morris front organization with United Nations NGO and US State Department links.]
1989 Nov 7: A week after the McGill conference Carlo was speaking on his "Cervical Cancer" designated sub-category at the Tobacco Institute's ETS Indoor Air Quality Conference. Most speakers on this list were members of the Holcomb (Aug 5 1989) Science Witness Team or known Tobacco contractors.
This appears to be a trail session with some SWT members test-lecturing on their designated subjects. 

1989 Nov 20: George Carlo and the Health and Environmental Sciences Group, staff have prepared the full Draft Protocols for the Newman Partnership Ltd, of Columbia, South Carolina. [The home address of Larry Newman] to hand on to Philip Morris.
Carlo's preamble lists reasons why Philip Morris should secretly fund both his 'Bias study' and the 'Health Scientist Survey'. He spells the obvious in transparent tobacco industry terms. Such pseudo-research was needed to counter....
He then outlined a series of four studies which would help the industry counter any likely regulatory measures which might follow the EPA release.
- The Japanese Hirayama study of lung-cancer among the wives of Japanese smokers, which had established that ETS (passive smoking) was a substantial risk factor in human lung-cancer among non-smokers.
- The same conclusion had been supported by the 1986 review of the ETS-lung cancer literature conducted by the National Academy of Sciences (NAS)
- And, in a few months (in early 1990), the US Environmental Protection Agency (EPA) was expected to release a series of quantitative risk assessments regarding ETS (passive smoking) and disease which may lead to national regulations restricting smoking in public places.
[This is a 183 page document with extensive details of the techniques, protocols, etc. The following is a partly paraphrased, and highly truncated version. The original document consists essentially of four parts:]
- Executive summary;
- Part 1 on the pseudo scientific research and its rationale,
- Part 2 which is a PR plan to promote the pre-ordained conclusions
- Appendicies
Executive Summary:
Specifically, three qualitative studies and one quantitative study of bias are described in this document. The three qualitative studies are interrelated and provide the basis for testing specific hypotheses regarding bias in the fourth study.
Although the studies build upon one another, each study is unique and it is anticipated that each will be published or otherwise disseminated independently as well as in combination.
- Minimum Standards Study — this study will identify good scientific practice standards [est. cost $131,350]
- NAS Study Review and Interpretation — to establish what interpretation the members of the NAS panel made. [est. $28,000]
- Scientist and Physician Opinions About the NAS Study and ETS — this study will determine what scientist and physician opinions are regarding ETS.[est. $89,000]
- Quantitative Study of Bias — this study will test the hypothesis that knowledge of the identity of the substance being evaluated creates a bias in interpreting the potential impact on human health [est. $90,000]
Part 1 is worded like many scientific research proposal's.
[The plan here proved to be more elaborate than those finally approved — but it clearly illustrates the concept of science-for-sale.]
Carlo's team recommended against peer-review because of the time constraints in releasing their anticipated findings before the EPA releases its Risk Assessment of ETS.
Would-be peer reviewers of the protocol (e.g. University of Michigan survey research professionals) should instead be hired to develop and implement the aspects of the studies considered to be most open to public criticism (e.g. development of interview instruments, development of sampling strategies).They also proposed getting exposure through shopping the results around via one-to-one meetings with scientists, without revealing that the tobacco industry funded the project.
[ Interpretation: They would peer-review the design protocols — not the actual research — and then still claim that the research has been 'peer reviewed'.]
Big Chill tactics on the EPA Researcher:
"When these studies are approved and "underway" , there might also be the opportunity to provide Dr Kenneth Brown, the EPA risk assessment contractor, with a briefing, prior to his submitting his final version to EPA.
Such a briefing with Dr Brown would both present an opportunity to influence his final version by providing him with new and relevant data, and alert him to the degree of sophistication applied to critiques of his work. This type of proactive peer review, or peer review with new data is highly unusual but effective. Knowledge that such intense scrutiny is being applied might cause him to think twice."
- [This attempt to chill the enthusiasm of EPA's principle scientist on the project didn't work in this case, but later they spent considerable effort in persuading Brown and his partner to do research work for them on the side.]
Publication in Scientific Journals:" Non-peer review journals such as American Scientist and the various state medical society journals usually accept for publication well written and interesting papers, but may require a local interest angle.
Both the peer review and non peer review journals routinely accept Letters to the Editor, and opportunities for this type of publication should also be sought. With respect to the most timely avenue to publication, letters to the editor and publication in The Lancet or a similar weekly medical or scientific news journal would be recommended."
Part 2 is a PR and lobbying plan for the studies; specifically to attack the risk assessment being conducted by the EPA. [Truncated text below]
Strategy.He also promotes publication in scientific journals, and...
"While much of the data to be generated in these studies have long term value and potential use, timing is of the essence in addressing the EPA risk assessments. Acceptance of the results by the scientific community will be gained both through rigorous briefing of key science groups and by publication in a peer reviewed journal. However, scientific publication should be viewed as a longer term goal since scientific peer review is time consuming and scientific journal publication backlogs are usually substantial.
[T]he overall strategy should involve recognition that the studies being done are intended to make a contribution to the literature and the science of assessing environmental health risks. This pertains especially to the minimum standards study and the quantitative study of bias. The value of these studies goes beyond the ETS issue as far as the science is concerned, and posturing these studies as broader than ETS is recommended.
[Rather than peer-review 'in advance', he recommends hiring a University of Michigan survey research group to avoid public criticism.]
In this way, scientific rigor is enhanced. This will also integrate into the process third parties who are likely to be viewed as objective by the general public and the scientific and public policy communities. Hopefully this will also enhance the publication of the results.
Dissemination of Results.
The results of these studies will be immediately useful in providing a science based critique of: the NAS review, the Surgeon General's opinion on ETS, and the forthcoming EPA risk assessments regarding ETS.
Based on these application purposes, there are a number of dissemination possibilities. In-person conferences, with the opportunity to present and discuss data tables and interpretations is most advisable, and likely to be most effective.
Finally, dissemination of these results to the general public should involve background media briefings, interviews and perhaps a video, perhaps in documentary format, describing the results of these studies in the context of the overall ETS health effects issue.Appendices:
Appendix 3 lists potential subcontractors. Maurice LeVois heads this list, followed by dozens of known corrupt or dubious scientists.
Cost estimates for different aspects:
- Phase I $ 14,400
- Phase II $ 116,950
- NAS Study Review $ 28,000
- Opinion Survey $ 89,000
- Study of Bias $ 90,000


George Carlo 2006 |
1989 Dec: /E The Tobacco Institute (TI) has circulated this list of available 'consultants' who are willing to give testimony at ordinance hearings, or before State assemblies, promoting the tobacco industry's line.
[It is important to realize that 'consultant' here means a scientist or academic paid to support the industry's position. They do not 'consult' in the normal meaning of the term, as in "providing genuine expert advice".]
Basically there are four categories of consultants, with lists of names. Every one of them is a well known long-term tobacco industry friend and "passive smoke denier". The memo says that the...
It gives the breakdown as being
- TI consults with 37 ETS and IAQ scientists: 14 are members of university or medical school faculties; 23 are professional consultants; 11 are exclusively expert on IAQ.
- Scientific disciplines include chemistry, toxicology, biochemistry, statistics, medicine, environmental science, biostatistics and industrial hygiene.
The memo also provides details of "Length of relationship," "How we use them," and...
- Academics: 14 academic scientists from institutions including the University of California; New York University Medical Center; Columbia University; University of South Carolina; University of Alabama; University of Maryland; Medical College of Virginia; Pace University; West Virginia University; Stillman College; New York Medical College; and George Washington University.
- ETS Consultants: George Carlo; Walter Decker; Thomas Golojuch; Gio Gori; Larry Halfen; Larry Holcomb; Alan Katzenstein; Maurice LeVois; Joe Pedelty; Jack Peterson; Barry Seabrook;.and David Weeks.
- IAQ Consultants: Peter Binnie; Bill Butler; John Drake; Jolanda Janczewski; D. Johnson; Gray Robertson; Jeff Seckler; Elia Sterling; Nancy Stone; Simon Turner; and Jon Yereb.
[IAQ Consultants are executives of a few indoor air testing companies who were paid to fake their findings to reduce the detected levels of smoke. They also travelled around the country promoting the fear of "sick building syndrome" which they said was caused by bacteria and viruses in air-conditioning systems — so blaming poor maintenance and the need for greater air-exchange rates.]
"Kinds of things they do"It then lists "What Have They Done lately", "Strengths", and "Limitations", Another section deals with special consultants for workplace smoking discussions with corporate CEOs to persuade them not to implement bans:
- Testify on federal, state and local smoking restriction and indoor air quality bills and regulations — explaining complex scientific information in straightforward lay terms.
- Appear on television and radio talk shows — often in debate formats — in areas where smoking restriction activity is underway.
- Assist the industry in responding to media reports by preparing critiques of adverse research.
- Help reassure allies that they are on solid scientific ground
- John Fox and Dennis Vaughn, two contracted labor lawyers
- Lewis Solomon, Dean of the UCLA Graduate School of Education (who attacks workplace smoking problems associated with productivity and absenteeism)


1989 Dec 6: Tom Borelli and Amy Millman circulate a memo within Philip Morris.
The EPA's Risk Assessment has been delayed and they have time to develop a new day-by-day strategy.
Another strategy we should pursue is to try and get a sympathetic scientist appointed to the health effects panel. New scientists are chosen each time an SAB panel is convened.
- Dec 11 - Lobbyists Mannett Phelps will have an EPA action plan ready.
- Dec 15 - The McGill University ETS Conference proceedings will be available for review.(The McGill Conference report must be distributed to scientists and policy makers.)
[Paul Dietrich of the Institute for International Health & Development had the job of publishing and distributing the procedings as booklets.]- Jan 10 - first opportunity to leak the EPA document to the press.
[They already had a confidential copy of the EPA's Draft Risk Assessment, but they needed to have some event to provide cover for the leak. Lisa Barrera had the inside connections within the EPA.]- Feb 15 - The EPA's Scientific Advisory Board will begin its review of the EPA's Risk Assessment.
[The SAB was reasonably independent — although the industry had a couple of their tame scientists on it.]- Feb 19 - 21 Toxicology Forum
[This was run by Coca-Cola executive Alex Malspina who also ran the ILSI. They believe that he is subject to PM/Kraft 'influence' if it comes in a brown bag. This forum gives them an opportunity to release scientific denial documents already prepared.]
We can use the McGill findings, and the information we have received that the group preparing the risk assessment was unable to establish a synergistic relationship between radon and ETS, nor were they able to prove a significant dose response. TI will undoubtedly respond to media inquiries but we should consider using the Newman Partnership creatively.
We need evidence that the scientists conducting research into, or reviewing the science of ETS, are less objective than they would be if they were dealing with another substance.
[A clear reference to the potential use of Carlo's "Bias study".]


1989 Dec 20: RJ Reynolds have prepared a comprehensive plan of action "Environmental Tobacco Smoke - Recommended Industry Program"
It incorporates the Philip Morris and Tobacco Institute plans along with many suggestions for Reynold's own scientific corruption activities. Carlo is mentioned as part of the Tobacco Institute Scientific Witness Team 

1989 Dec 31: /E In 1989 Carlo received two Philip Morris payments ($70,000 + $60,000) for his papers proving that epidemiology is wrong, and that anti-tobacco scientists are biased and so produce distorted results.
Two different HES staff 'researchers', Kelly Sund and Rebecca Steffens, are listed as co-authors on the paper. Kelly G Sund's name is on the draft, and Rebecca Steffens [later Jenrow] appears on the final document.
Kelly Sund was a faithful employee who acted as a travelling companion, general factorum and the only employed co-researcher to Carlo until now. She is never listed as having any biomedical qualifications, although she was also co-author on the dioxin-spill study of the Melbourne (Australia) water supply in this year.
Maurice LeVois also managed to take $25,000 from Philip Morris for similar work at the same time. He had already begun to work out of San Francisco with another Californian shonk and tobacco lackey called Max Layard, but he is still on record as running "HES-West".
1990: Carlo's Health and Environmental Sciences Group, Washington, DC published a report Industry Task Force on 2,4-D Research Data. Backgrounder on the uses of 2,4-D.
Phenoxy herbicides include 2,4-D, 2,4,5-T (banned for all uses in the United States in 1978), 2-methyl-4-chlorophenoxyacetic acid, and other related compounds.
2,4-D is one of the most commonly used broadleaf herbicides in North America. It is widely used in agriculture on crops such as wheat, corn, oats, rye, barley, sugar cane, and sorghum. It is also used on range and pastureland, in forestry, on rights-of-way, and on lawns and other turf, such as golf courses.

This document appears to have been superceded by

1990: Tobacco Institute's Confidential notes on Public Smoking Hearings:
Consultants:
Who they are. How long have they been working for TI. What they do.
BackgroundThe document lists the following activites of these consultants.Who:
- TI consults with 37 ETS and IAQ scientists: 14 are members of university or medical school faculties; 23 are professional consultants; 11 are exclusively expert on IAQ.
- Scientific disciplines include chemistry, toxicology, biochemistry, statistics, medicine, environmental science, biostatistics and industrial hygiene.
- Academics: 14 academic scientists from institutions including the University of California; New York University Medical Center; Columbia University; University of South Carolina; University of.Alabama; University of Maryland; Medical College of Virginia; Pace University; West Virginia University; Stillman College; New York Medical College; and George Washington University.
- ETS Consultants: George Carlo; Walter Decker; Thomas Golojuch; Gio Gori; Larry Halfen; Larry Holcomb; Alan Katzenstein; Maurice Levois; Joe Pedelty; Jack Peterson; Barry Seabrook; and David Weeks.
- IAQ Consultants : Peter Binnie; Bill Butler; John Drake; Jolanda Janczewski; D Johnson; Gray Robertson; Jeff Seckler; Elia Sterling; Nancy Stone; Simon Turner; and Jon Yereb.
- How we use them
- Prepare and deliver testimony.
- Conduct briefings with legislators; regulators; lobbyists; and coalition allies.
- Conduct two ETS and one IAQ media tours per month.
- Conduct empirical IAQ research.
- Monitor scientific developments on ETS and IAQ.
- Prepare articles for publication.
- Submit letters to editors of scientific and general-audience publications.
- Attend and report on scientific conferences.
- Kinds of things they do:
- Testify on federal, state and local smoking restriction and indoor air quality bills and regulations — explaining complex scientific information in straightforward lay terms.
- Appear on television and radio talk shows — often in debate formats — in areas where smoking restriction activity is underway.
- Assist the industry in responding to media reports by preparing critiques of adverse research.
- Help reassure allies that they are on solid scientific ground.


| Herbicides, dioxins and furans |
|---|
When herbicide companies maintain that their studies of "TCDD" herbicides did not produce tumours, etc. etc.be aware that they may be playing with words:
|
Some time in 1990 the OTA's Agent Orange committeee was dissolved.
1990 Feb: - Mar The American Paper Institute and the Chlorine Institute were organising to challenge the early EPA findings that dioxins were extraordinarily dangerous.
[Note: These ideas rested substantial on the research done by Dow Chemical's own Dr Richard Kociba in 1978]
They therefore hired a consulting firm, ChemRisk, to set up a 'independent' "Pathology Working Group" to review the Kociba work.
[ChemRisks was actually a division of McLaren/Hart and run by the well-known science lobbyist, Dennis Paustenbach.]
- Dr Robert A Squire — from Johns Hopkins Uni [a later Carlo associate] supervised the project.
[His name isn't on the published report] - Dr Denis Paustenbach — the science lobbyist/President at ChemRisk. He later sifted to Exponent Inc.
- Russell E Keenan — ex-EPA, then with ChemRisk. He is now with Integral Consulting working for the Maine Pulp & Paper Assn.
- Richard J Wenning — ChemRisk, later sediment rehabilitation specialist [Now at ENVIRON]
- Alan H Parsons — a junior toxicologist at ChemRisk
In the light of these new 'independent' findings the API wrote to the EPA claiming:
"All of the Agency's analyses are now out of date in light of the significant new evidence showing that the risks of dioxin has been overstated."
[ChemRisks was a subsidiary of McLaren/Hart and Dennis Paustenbach was its founder and president. He's also a well-known science-for-sale operator.
The above illustrates why scientists are wary of letting data or experimental evidence get into the hands of special interest groups who are embarking on data-mining expeditions.]
See also PubMed and 
1990 Feb 8: Larry Holcomb of Holcomb Environmental Services , who is organising the 'independent' Scientific Witness Teams (SWT) for the Tobacco Institute (giving evidence for the company during lawsuits, or at government hearings) is reporting to to his TI controller Kay Thomas about the first year of operations:
For the last 4 months of 1989 (when the program first began) it has cost approx. $42,050 divided up as follows:The funds have been distributed amongst five experts; [George] Carlo, [Walter] Decker, [Larry] Halfen, [Maurice] Levois and [Joe] Pedelty .
- Cervical cancer and cancers other than Lung Cancer, $21,800;
[This was the subcategory allocated to Carlo and LeVois]- Cardiovascular $9125
[Decker and Halfen];- Biomarkers, $2775
[Decker];- Epidemiology, Statistics/ Methodology $2100;
[Also allocated to Carlo and LeVois]- Risk Assessment, $6250.
[Holcomb and Pedelty]
When we started, I asked Jack Peterson to indicate on his billings, any special subcategory for which he was billing. I telephoned him recently and he said he didn't believe he had billed for anything special.


1990 Feb 19: -21 The annual Winter Toxicology Forum (run by the ILSI but not by the usual Alex Malaspina) was held at Lowes L'Enfant Plaza Hotel in Washington
[Lowes is the hotel chain owned by Lorillard, The Toxicology Forum was a dubious operation which ran an alternate series of conferences to that of the Society of Toxicology. Most of the shonkly scientists were prominent in both organizations. ].
George Carlo wasn't at this conference. However his partner Maurice LeVois was... and it was loaded with tobacco scientists:
- Maurice LaVois, Ph.D., HES-WEST
#4 Emborcodero Center Suite 2000 San Francisco, CA
[Note that LeVois is still using the Health & Envtronmental Sciences business name] - Maxwell Layard, Ph.D. President Layard & Associates ,2242 San Antonia Avenue Alamda, CA
[This is LeVois's new partner] - Dr Ian Munro, Director, Canadian Center for Toxicology, 645 Gordon Street Guelph,Ontario Canada
[A later Carlo partner]


1990 Feb 23: [In a July 20 1994 memo] from William Sanlour, Policy Analyst with the EPA to Director of the Characterization and Assessment Division, David Bussard.
The memo notes that on February 23, 1990, Cate Jenkins, a PhD chemist at EPA was working on a project to develop regulations to control hazardous waste, had come across evidence of fraudulent science. She sent a memorandum to the EPA Science Advisory Board entitled "Newly Revealed Fraud by Monsanto in an Epidemiological Study Used by EPA to Assess Human Health Effects from Dioxins"
In February 1990, Dr Cate Jenkins, a chemist at the U.S. Environmental Protection Agency, wrote to the EPA Science Advisory Board that there was evidence that the Monsanto studies were fraudulently done and that if the studies had been done correctly, they would have shown the connection between dioxin and cancer in humans. This accusation received considerable press attention.
In August, 1990 EPA decided to launch a criminal investigation of Monsanto. Amid a furor of publicity and cries of foul and intensive lobbying by Monsanto the criminal investigation went on for two years. However, despite the government's assurances that it would "investigate any allegations of fraud and, if appropriate, evaluate the full range of enforcement options" it did nothing of the kind. Instead it investigated and illegally harassed the whistleblower, Cate Jenkins.
In August of 1992, EPA quietly closed the criminal investigation without ever determining or even attempting to determine if the Monsanto studies were valid or invalid, let alone fraudulent. However, the investigation itself and the basis for closing the investigation were fraudulent.
Jenkins' harassment was subsequently halted by order of the Secretary of Labor. The veterans were able to use her report to obtain increased Agent Orange benefits from Congress for Viet Nam cancer victims. Recent EPA reports say that there is now convincing human evidence of the carcinogenicity of dioxin, in contradiction to the Monsanto studies.
This investigation has left the unanswered question: did Monsanto manipulate their studies in order to play down the danger of dioxin so as to reduce their liability to the Viet Nam veterans? And it has raised two more questions. Are top EPA officials more concerned with protecting their employment prospects with the industries they regulate than in protecting human health and the environment? And, are EPA law enforcement officials being used as an internal KGB to silence dissent?
See actual memo and a discussion paper 
1990 March: The Georgia Regional EPA Administrator struck down the State standard for dioxin after hearing that Vernon N. Houck, director of the Center for Environmental Health and Injury Control at the Centers for Disease Control, had admitted to the chair of a Congressional subcommitteee that he had extracted large amounts of his report from dioxin studies prepared by the paper industry.
1990 Mar 7: The Chlorine Institute ran a Plant Operations Seminar in Houston. The paper, "Scientific Research on the Health Effects of Dioxins in the Environment: A Review and Summary of the Issues" by George L. Carlo, Chairman, Health & Environmental Sciences Corporation is made available as part of the proceedings of that seminar (but not separately).
The paper says it was "prepared with the assistance of Health and Environment Sciences Group and Ketchum Public Relations personnel."
[ Ketchum Public Relations was the scientific lobbying firm later credited with finding Carlo as the head of research for the Cellular Telephone Industry Association]
The paper industry's newsletter, the "Alkine Paper Advocate", put excerpts from Carlo's lecture up on the web. In these excerpts Carlo had claimed:
Many scientists now believe that low level exposure to dioxins does not represent a serious public health hazard.
While some studies purport to show an association between exposure to dioxins and cancer in human, those studies are widely criticized and not widely accepted by the scientific community.
1990 Apr 4: Newman Parnership Ltd (NPL) has sent a new proposal to Tom Borelli at Philip Morris: "Variations on Varela".
| Explanation: Luis Varela had been a graduate student at Yale in 1987 working on a PhD dissertation on the health-effects of ETS under his Yale supervisor, Dwight Janerich. Varela died suddenly before the dissertation was published, but somehow draft copies and some primary data found their way into the hands of the tobacco industry. They reviewed the raw data; concluded that it supported their case, and hurridly published sections of the dissertation under the Varela name as evidence there was still serious doubt that ETS was harmful to non-smokers... and subsequentially accused the EPA of having deliberately ignored scientific evidence that was contrary to their anti-smoking position. It was pathetic last-minute attempt to counter the EPA's release of its Class A Carcinogenic Risk Assessment. However, as NPL pointed out in this memo it was exploitable. Unfortunately, Janerich disagreed with the tobacco industry's interpretation of Varela's work and was about to publish his own paper using the same data. He believed that it showed causal links between ETS and lung-cancer. |
The NPL suggested to Philip Morris that they have a tame independent scientist check the Yale material, and if possible, provide support for the tobacco industry's interpretation.
If the findings proved to be favourable, they would then give the story as an exclusive to Lawrence Altman of the New York Times. Alternately, they could run the material as an advertisement in the New York Times under the caption:
Tobacco didn't fund the study,A range of ploys are discussed in this memo for further use of the Varela material including mailing it to science and policy makers (along with the McGill Uni booklet) and
Tobacco didn't write the story.
Sometimes truth just won't be denied
When the Carlo study on bias is completed, Varela could be incorporated into plans for PR.


1990 May: Nufarm Australia:
Australian Greenpeace revealed that Nufarm Chemicals [Herbicide manufacturers owned indirectly since 1982 by Dow] was discharging illegal levels of chlorinated phenols and high levels of dioxins and furans into the sewerage system at Werribee in the Melbourne water catchment area.
The local EPA had been warned by their own staff about dioxin discharges from Nufarm as early as 1978. The effluent was being discharged illegally from the plant into an open drain behind the factory, and water flowing from the drain into the Cherry Creek/Lake System.
In December 1978 the Victorian government EPA's principle Water Quality Officer wrote in regard to unlicensed discharges from Nufarm into the creek:-
"A by-product of 2,4,5-T manufacture, dioxin or TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin), has received considerable publicity recently because it causes serious acne and has been linked with cancer and birth deformities. I would suggest that the analyst be contacted to ascertain if the samples were analysed for dioxin." (EPA Memorandum 28/12/1978)In a handwritten note attached to the internal memorandum, it is stated that the Department of Agricultural Laboratories were contacted on the matter but they did not have facilities to carry out the analysis. The question of dioxin contamination then appears to have been dropped.
"I anticipate... that a licence application for such discharges would be refused by the Authority (or MMBW [Metro Water Board]) because of the obvious threat to aquatic life and wildlife, and the possible health risk to humans."
Further tests in 1986 showed that that 2,4-D (the major pesticide produced by Nufarm) contained dioxins
EPA Draft Risk Assessment on ETS.
It also reported its intention to label passive smoke as a Class A Carcinogen
(The friends of the tobacco industry had already leaked this information.)
1990 May: The EPA circulated to its Scientific Advisory Board [SAB] and other interested parties the review draft of a report on passive smoking [ETS] entitled "Health Effects of Passive Smoking: Assessment of Lung Cancer in Adults and Respiratory Disorders in Children.".
At the same time, letters were exchanged between the Tobacco Institute and Republican Congressman Thomas J Bliley [R-Va who was known as "Mr Tobacco" — a "tireless champion for the tobacco industry"]. These were later leaked and exposed the tobacco industry's involvement in an attempt to force Dr David Burns out of the EPA's SAB [Scientific Advisory Board] on ETS after he had been invited to become a member. 
The tobacco industry and Rep Bliley claimed that Burns was 'biased' and one senior tobacco executive labled him a "squeaky-clean ignorami (sic) well known for his militant anti-smoking beliefs."
The exposure of the Bliley correspondence resulted in a furore, and had the result of seeing Burns reappointed along with a couple of other independent scientists who were also not under the industry's control.
[The Washington Legal Foundation also objected to the SAB process] 

It was a major crisis for the industry at a critical time, and they were forced to lay low. One Philip Morris executive was furious at the botched outcome:
Regardless of the ultimate "findings" of the group, confirmation of the "science" alone will have devastating effects on a worldwide basis.They decided to make the best of a bad situation by preparing for the actual release (later the same year) by having:
The industry, as such,, has no credibility and no forum, for fairness. The "'lobbying" against Dr Burns is viewed by the media, the EPA, etc., as yet another tactic typical of the industry and has been characterized as desperate. The lack of knowledgeable, credible "white coats" [undercover secret supporting scientists] willing to speak for the industry is particularly debilitating.
A combination of Parrish/Borelli and any credible scientists who will enter the fray under any terms (background, not for attribution, etc.) are perceived to:be the best, if not the only, communicators with any chance.to achieve any semblance of balance to the story
[Clearly most of their 'witnesses' and regular 'consultants' were not prepared to take a public position after the Bliley leak, so PM had to fall back on the two spokesman/executives.]
- Steve Parrish make a presentation and/or write a bylined article which would be fed to any media outlet that would run it.
- Dr Edward J Burger, Jr, Director of the Institute for Health Policy Analysis, and a professor at Georgtown would be asked to make a public protest as an independent scientist.
- Peter Huber of the Manhattan Institute (the originator of the "junk-science' phrase) would possibly "write an op-ed linking ETS to pathological science" targeted at the Washington Post.
Obviously, as a reliable science-for-sale entrepreneur, Carlo would be called upon. He was one of their most useful recruiters and trainers of "White Coats" [scientific lobbyists] and had just completed a study to prove scientists and doctors are 'biased' against the tobacco industry. He is therefore prominently in their plans. Their check points include:
- ... add the bias pitch to all editorial board briefings and meetings with journalists.
- write a op-ed piece for them and act as an independent spokesman in a planned attack on the EPA's reformed Scientific Advisory Board.
- Publication of Carlo op-ed and coalition development (in the works)
[He is also to help in the creation of an anti-EPA coalition (recruiting other industry groups).]- Media training for Borelli, Carlo, et al. tenatively scheduled for November 8 & 9, Washington
[Philip Morris had irregular media training sessions organized through PR firm Burson-Marsteller].


[ Steve Parrish was a top lawyer-executive at Philip Morris USA (Exec VP Scientific Corporate Affairs) in charge of the scientific lobbying effort, and Tom Borelli (Director Science Issues) and Vic Han (Worldwide Scientific Affairs) were his executives in charge of the day-to-day disinformation and corruption campaigns.
The Nov 8-9 media training sessions were to be followed by a "Carlo Strategy Meeting" at the Burson-Marsteller offices in Washington.]


[They managed to stall the final Risk Assessment on ETS until Jan 7 1993]
1990 May 7: Nufarm Australia:
Greenpeace activists arrived at the Nufarm chemical company, taking control of the plant's effluent discharge into the sewer, and creating a news event which the EPA could no longer ignore.
The [EPA's] analysis results were released on May the 10th 1990 [and] the EPA tests found furans (2,3,7,8-TCDF) and other toxic polychlorinated dioxins.However, in July 1990, Nufarm received a licence to pollute, thereby legalising their dioxin discharge.
Dr Brian Robinson, Chairperson of the EPA, said that as there are no safe levels for dioxins and furans,"they should not be entering the sewerage system at all. They are dangerous in the sense that they can cause harm to the environment in even very small levels...Our major concern is that these materials bioaccumulate. Why take the risk if we don't have to?"
Production of 2,4-D at Nufarm was halted and the company required to conduct an environmental audit, the first of its kind in Australia. [Source: The Age, May 1990]
The discharge sample was tested by National Analytical Laboratories and found to contain 1.4 parts per billion [or 143 parts per trillion] of the furan 2,3,7,8-TCDF. As a comparison, 0.038 parts per trillion in water is enough to start killing fish. (Mehrle PM. et al. 1988)
Nufarm's effluent also contained chlorophenols, a group of chemicals that are the precursors for the manufacture of 2,4-D. Several different types of these chemicals were found, including dichlorophenol and trichlorophenol. The Greenpeace samples contained up to 5,000 parts per million of these substances, 100 times Nufarm's allowed limit as set in their old Trade Waste Agreement (NAL Report, Greenpeace, April 1990).

1990 May 31: The Washington Post surprised the world with the headline, "Scientists Temper Views on Cancer-Causing Potential of Dioxin."
The story, by Malcolm Gladwell, said,
"Dioxin — the chemical that forced the evacuation of Love Canal, sparked a wave of lawsuits over Agent Orange and became notorious as the most potent carcinogen ever tested — may be far less dangerous than previously imagined, according to new scientific evidence."Gladwell's "chorus" consisted of quotations from four scientists and he neglected to mention that three of them were consultants paid by the paper industry — an industry that had serious dioxin discharge problems associated with chlorine bleaching.
[This followed the ChemRisk revision of Dow's earlier Kociba study]
"Enough experts have joined the revisionist chorus that some scientists consider a softening of the government's stance toward the chemical inevitable."

1990 June: Nufarm Australia:
Following the public clamour about a dioxin spill into the water supply of Melbourne, Australia, Carlo's HES team were asked to study the health risks. They wrote in one abstract:
During June and July 1990, surface soil samples were taken in the Melbourne metropolitan area and analysed for phenolic compounds, chlorinated herbicides, polychlorinated dibenzo-para-dioxins (PCDDs) and polychlorinated furans (PCDFs).[It goes without saying that this report does not mention that Nufarm was a subsidiary of Fernz in New Zealand, which itself was a subsidiary of Dow Chemical and that George Carlo was promoted on various occasions as "Technical Director" of Fernz.]
[It doesn't say who was skilled enough to perform these tests, because the Victorian water experts clearly weren't.]
A surface soil sample from a Werribee Farm Treatment Complex paddock, where cattle graze on land that is used for filtration of sewage, was also taken and analysed. No phenolic compounds or chlorinated herbicides were detected at the parts per billion detection limits in any of the samples.
[Parts-per-billion limits are no longer considered to be refined enough for endocrine and similar suspect mutagenic measurements. They now need parts-per-trillion.]
PCDDs and PCDFs were detected in both industrial and nonindustrial sectors of the Melbourne metropolitan area, as well as in effluent from Nufarm Limited, an agricultural chemicals manufacturer in Laverton North, in similar concentrations (toxic equivalents in the parts per trillion range). These concentrations were consistent with background levels identified in other major urban areas.
There was no evidence of the Nufarm effluent fingerprint in any of the background soil samples analysed. The fingerprint profile of the sample taken from Werribee Farm, although showing traces of the Nufarm effluent fingerprint, was clearly distinct from that effluent fingerprint and consistent with the fingerprint identified in the background soils. The impact of the Nufarm effluent on the area, therefore, was considered insignificant.
[This claim to be able to perform a 'fingerprint profile' is rather dubious when the work is being conducted by an epidemiologist, his secretary and a lawyer.

1990 June: The EPA released two draft reports into the public domain
• "Health Effects of Passive Smoking"
• "ETS: a Guide to Workplace Smoking Policies"
The EPA's Risk Assessment on Passive Smoking (ETS) claimed that about 3,800 non-smokers died each year from tobacco-caused lung-cancer and other health problems.
The Tobacco Institute and the cigarette companies were preparing an all-out blitz on the top newspapers in the USA (initially 30 — later top 75) to counter the EPA claims. The plan was to run a series of editorial board briefings utilizing Tobacco Institute, Philip Morris and RJ Reynolds lobbyists.
They hired Carlo's partner, Maurice LeVois to provide scientific back-up to their claims. He was promoted to newspaper editors as an independent expert who could show they why the EPA was wrong.
[LeVois had no biomedical qualifications whatsoever].
They also listed national columnists who they consider "fair" or "favourable" to their cause who were to get special material, and the Institute's scientific consultants had distributed op-eds to their local newspapers, incorporating Philip Morris's McGill University closed conference reports and other material. Through commissioned letters-to-the-editor and other materials, they were attacking the EPA's science, and bolstering their claim that the EPA was politicized and biased against the tobacco industry.
This package of documents includes the
- plan of attack on editorial boards,
- lists of the major newspapers
- the allocation of lobbyists,
- sample letters to be sent
- a long C/V of Maurice LeVois.
We also stand ready to present this scientific evidence to the media. To that end, we would be happy to meet with you and your editorial board to discuss these issues. At your convenience, Maurice LeVois, Ph.D.,an expert in epidemiology and risk assessment, and I would like meet and cover these issues.
Dr.LeVois' expertise has been called on by the American Red Cross, the Centers for Disease Control, the Veteran's Administration and others (copy of curriculum vitae enclosed). The Tobacco Institute calls on his expertise in his capacity as a consultant.
I will telephone you later this week to determine your interest, and, I hope, to set up a meeting time.


1990 June: A Tobacco Institute has also circulated this Issues Paper to its Regional and State Divisions. It provides the offical industry line on dozens of topics, including those dealing with "Tight Building Syndrome", "Sick Building Syndrome" and Indoor Air Quality (IAQ) issues
It also lists full-time consultants who are available to help the various State lobbyists fight any attempts at public smoking bans. They have two categories of consultant:
Environmental Tobacco Smoke (ETS)[Every one on this list is a well-known, long-term science lobbyist for the tobacco industry. These were all considered 'safe' scientists for the State or Regional Directors to contract as witnesses, etc. at Local Ordinance or State Assembly hearings.]
- Lawrence Halfen — Environmental Consultations Inc, Grand Rapids, MI
- Larry Holcomb — Holcomb Scientific Services, Olivet MI
- Walter J Decker — WJ Decker Toxicology Services, El Paso TX
- George Carlo — Washington DC
- Maurice LeVois — San Francisco, CA
- David Weeks — Boise ID
- Jack Peterson — Peterson Associate, Brookfield WI
- Joe Pedelty — Holcomb Environmental Services
Scientific Witness Teams on Indoor Air Quality
- Gray Robertson — Healthy Buildings International (HBI), Farifax VA
- Simon Turner — (as above)
- Jeff Seckler — (as above)
- Peter Binnie — (as above)
- Jolanda Janczewski — Consoliated Safety Services, Oakton VA
- Nancy Stone — (as above)
- Jon Yereb — (as above)
[Both of these companies was engaged in commercial indoor air quality analysis under a scheme where they were paid by the TI to discount smoke as a source of building problems. They were also available as legislative witnesses and for media tours, to discount smoke as an environmental health issue. ]


1990 Aug: The EPA decided to launch a criminal investigation of Monsanto following evidence that the Monsanto [dioxin - Agent Orange] studies were fraudulently done and that if the studies had been done correctly, they would have shown the connection between dioxin and cancer in humans. This accusation received considerable press attention. [The action was also inexplicably dropped in 1992, and Dr Cate Jenkins, the whistleblower was repremanded for making the accusations. This was later reversed after many court cases.]
See also the simultaneous publication of the ChemRisk Report of Dioxin - Related Issues by Russell E. Keenan, PhD, to the Arkansas Pollution Control and Ecology Commission

1990 Oct: / E Nufarm Australia:
Around this date the "Assessment of dioxin-related health risks for the Melbourne metropolitan area" by George L Carlo, Kelly G Sund, James Baller. was sent to the Victorian state government.
[Only published later in June 1993 in the Australian Journal of Public Health.]
Abstract: A community health risk assessment was conducted during 1990 in Melbourne for polychlorinated dibenzo-para-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) released in Laverton North by Nufarm Limited, an agricultural chemicals manufacturer.
This risk assessment incorporated current scientific knowledge into hazard identification, dose-response assessment, exposure assessment and risk characterisation sections, according to the four-step framework used by the United States Environmental Protection Agency.
The hazard identification showed that health effects are unlikely to result from general population exposures to PCDDs and PCDFs. The dose-response assessment supported a safety-factor approach for PCDD and PCDF risk assessment. The exposure assessment incorporated exaggerated assumptions to estimate both total daily exposure (203 pg total toxic equivalents of PCDDs and PCDFs, or 2.9 pg/kg body weight) and daily exposure attributable to Nufarm (56.4 pg, or 0.80 pg/kg body weight) under a worst-case scenario. The risk characterisation section found that exposures under 20 pg/kg body weight per day should not induce the aryl hydrocarbon hydroxylase system, which appears to be the starting point for PCDD and PCDF toxicity.
We concluded that the general population exposure to PCDDs and PCDFs in Melbourne was within the range of acceptable daily intakes used by European and Canadian governments and the World Health Organization, as well as within the range of acceptable daily intakes derived using current scientific knowledge.

When Carlo's report on the Melbourne water supply was released to the media in Australia he was promoted as an "American government expert" on dioxin who had conducted an "independent audit" of the community health risk of the water supply — and found no problems.
[Greenpeace didn't accept either the man or his conclusions and continued to harass the Victorian State Government until a second American dioxin expert — Carlo's OTA associate Michael Gough — was sent out to confirm Carlo's findings.
To be fair, in the light of current knowledge there probably wasn't a problem (which doesn't excuse the cover-up)
In Carlo's report there is no mention of the fact that he was
- a consultant to Dow Chemicals and part of their flying circus of misinformers.
- involved with the American VA/Military in their Agent Orange coverups,
- still working for the Chlorine Institute as a consultant
- still working for the Chemical Manufacturer's Association
- 'Technical Director' for NuFarm's parent company Fernz New Zealand (itself a Dow subsidiary)
1990 Oct 9: Carlo and his Health and Environmental Sciences Group present a preview of the Philip Morris "Health Scientist Survey" at the New Orleans Annual Meeting of the Society for Risk Analysis. It is now called "Potential value judgement influences among scientists asked to evaluate the hazards of dioxin, radon and ETS


[This preview must have been considered less than satisfactory by Philip Morris, since the actual release of the studies was delayed for a year. Carlo claims that work on this and the "Bias Study" was done on it in 1990 and 1991 — which is a remarkably long time for a couple of telephone surveys.
Of course, when the Banbury Conference (below) blew up, they may have decided that Carlo's name as an 'independent scientist' was not a credible concept to sell to the media.]
1990 Oct 22: - 24: Banbury Center Conference
The Banbury Center Conference on dioxins was held over these three days at Cold Stream Harbour. It had been organised by the Chlorine Institute to discuss the EPA's use of Kociba and other research in their risk-characterisation. Independent scientists were not invited, and there were only a few from the EPA, despite it being organised as a joint industry/EPA project..
In The Dioxin Wars, author Robert Allen (from Sunday Times, UK investigative team) says
The Chlorine Institute then stood back and let Banbury officials organise the meeting and select the participants, requesting only that their consultant Michael Gallo attend as an observer for the Institute.A later examination of this fiasco explained:
The meeting, which was held in October 1990, was attended by 38 dioxin specialists. There was no general consensus among the scientists who disagreed on many points. Some felt that the latest data on dioxin merited a fresh assessment by the EPA and even suggested that the agency had over-estimated the risks from exposure. Ellen Silbergeld, a toxicologist at the University of Maryland, rejected this notion.
Confident that it could present the conference in a favourable light for industry, the Chlorine Institute employed a public relations company, Edelman Medical Communications to prepare a press pack.
The package contained a press release from the Chlorine Institute, statements from three scientists, and a background paper by George Carlo who had also attended the meeting as another observer for the Institute.
The press release had been carefully drafted and dioxin had been carefully detoxified. The Institute announced that a consensus had been reached on several key issues, specifically that a threshold level for dioxin existed, below which these chemicals posed no general risk to human health.
Carlo, in his paper, announced that the meeting had 'reinforced the notion that dioxin is much less toxic to humans than originally thought'.
In 1990 the Chlorine Institute, a chlorine industry trade group with members such as Dow Chemical, Du Pont, Georgia-Pacific, International Paper, and Exxon Chemical Co, organised a conference of dioxin scientists at the Banbury Center.
Also in attendance was George Carlo, consultant to the Institute. The Institute hired Edelman Medical Communications to publicise any conference outcome that was to the Institute's advantage.
Following the conference, Edelman sent out a press packet with a background paper put together by Carlo, Edelman and the Institute, claiming that the conference had reached a consensus that dioxin was "much less toxic to humans than originally believed."
This outraged some of the scientists present who had not reached this conclusion and who felt that they had been manipulated by the Chlorine Institute (Roberts 1991a).
According to the magazine Chemistry and Industry, the Institute was merely coordinating a "public outreach program" to "capitalise [sic] on the outcome" of the conference. (Quoted in Montague 1991b)
Indeed the industry was able to use the supposed Banbury conference consensus together with the rat tumour re-count to get some States in the US to loosen dioxin standards for discharge of wastes into waterways, below those set by the EPA (Bailey WSJ 1992)
. [They were technically able to do this if they could support their choice of standards scientifically.]

Later more detailed explanations emerged:
"Flap Erupts Over Dioxin Meeting"It now appears certain that the meeting had only reached agreement on a suggestion that the EPA review the way it assessed dioxin's carcinogenicity (in view of the Kociba study review). However the PR firm, Edelman Medical Communications, then put out an exaggerated press release...
When the Chlorine Institute, an industry trade group, approached Banbury director Jan Witkowski early last year about holding a dioxin meeting; he had no inkling it would turn out differently from the 15 or so other meetings the center runs each year, some of which are also sponsored by industry.
The Chlorine Institute lined up Robert Scheuplein of the FDA to run the meeting. He then asked Michael Gallo, a toxicologist at the Robert Wood Johnson Medical School in New Jersey, and Cornelius A. van der Heijden, a regulatory official in the Netherlands, to chair the meeting with him. From then on, the Chlorine Institute studiously kept itself out of the picture, say both Witkowski and the meeting organizers.
The three organizers picked all participants, with one exception: George Carlo, who was invited as an observer for the Institute at its request. Carlo, an epidemiologist and lawyer who heads the Health and Environmental Sciences Group in Washington, DC, is a regular consultant to the Institute.
... with a background paper written by Carlo that none of the organizers or participants had seen — which claimed among other things that the Banbury meeting "reinforced the notion that dioxin is much less toxic to humans than originally thought."The cover letter from Edelman also claimed this was a "consensus conference." It was also later claimed that a disclaimer saying that "the opinions expressed were not those of the conference as a whole" which was originally in the letter, had been deleted.
When Ellen Silbergold, a toxicologist at the University of Maryland, saw a copy of the release in January, she blew her stack and began writing letters.
Events then took a bizarre twist when Carlo, who received a copy of Silbergold's letter, called Witkowski [Banbury Center head] to say he had not written the paper after all and had no idea how his name ended up on it.
Carlo has since launched a massive telephone campaign — calling the participants and this reporter repeatedly — to clear his name. Carlo concedes that he did work with Edelman and the Chlorine Institute in developing the paper but asserts, nonetheless, that "No one has the right to put someone's name on a document."
Nancy Turett, senior vice president at Edelman, admits to putting Carlo's name on the paper — she says because he was so extensively involved in drafting it. " "The end product is very much a reflection of what Dr Carlo thought should be in it,"" says Turett.


target=tobdocs>
 [What is most interesting here is the failure of the media to correctly report on this conference. Most completely ignored the fact that Michael Gallo was also a chemical industry consultant, and he played an important part in this conference.]
[What is most interesting here is the failure of the media to correctly report on this conference. Most completely ignored the fact that Michael Gallo was also a chemical industry consultant, and he played an important part in this conference.]
| Carlo's earlier statements |
|---|
| The paper industry November 1990 newsletter "Alkaline Paper Advocate" has an article "Some Credible Information on Dioxin" which quotes a literature review prepared for a March 1990 seminar of the Chlorine Institute, presented by George L. Carlo, Health & Environmental Sciences Corporation.
The title of that review is "Scientific Research on the Health Effects of Dioxins in the Environment," and it is available as part of the proceedings from that seminar for $15 from the Chlorine Institute, 2001 L St., NW, Suite 506, Washington, DC 20036. The article quotes from Carlo's review:
|
1990 Nov: 8-9: Tobacco: George Carlo is receiving media training through Burson-Marsteller in preparation for him acting as as independent scientific critic of the EPA's Scientific Advisory Board Risk Assessment of ETS (see above, May 1990)
[The Banbury Conference story hadn't broken in the general media at this time]
1990 Nov 10: Tobacco: Philip Morris's Washington strategy meetings with Steve Parrish, Tom Borelli, Amy Millman, Vic Han (misspelled Hahn), Robert Pages and George Carlo. It was a planning meeting to attack the EPA's Risk Assessment. It also lists as attendees:
- Barracuda - Possibly a code name for an adviser to PM - maybe a creative executive from Leo Burnett advertising.
- Herbolberbol - Clearly a nickname. This is probably Bob Herbolsheimer of lobbyist Mannett Phelps, Rothnber & Phillips.
- Burson - It was ccd to Gary Auxier, the Washington strategist of Burson-Marsteller,.
- Shooks - generic for unnamed Shook Hardy & Bacon lawyers.


1990 Nov 30: [Source EPA June 1994 memo] Another Malcolm Gladwell article in The Washington Post, "Greenpeace Digs Deep Into Dioxin Debate"
Greenpeace issued a detailed 44 page critique of the Monsanto studies by Joe Thornton entitled "Science for Sale" which repeated [Dr Cate] Jenkins' [of the EPA] allegations and added several more, followed by a petition to EPA to investigate the [Monsanto dioxin] studies, and held a well publicized press conference.
The Washington Post reported that: "The EPA said yesterday it is looking into Greenpeace's charges."
1991 Jan 24: A comprehensive study of US chemical workers exposed to dioxin in the course of their work at least 20 years earlier was published in the New England Journal of Medicine. The 13 year study was carried out by Marilyn Fingerhut and her colleagues at the National Institute for Occupational Health and Safety on over 5000 men who worked at 12 different factories between 1942 and 1984.
It found that the workers had a 15% higher death rate from cancer than the US average. Those exposed to low levels of dioxin (about 90 times background levels) had no statistically significant increase in cancers, whilst those exposed to high levels (about 500 times background levels) were 50% more likely to die of cancer (Roberts 1991b, p. 625).

[The editorial in the NEJM said about dioxins that "The hypothesis that low exposures are entirely safe for humans is distinctly less tenable now than before." ]There are three different and highly diverging reports of the same NEJM article:
- New York Times story by Warren Leary — "High Dioxin Levels Linked to Cancer."
- Wall Street Journal article — "U.S. Study Suggests Exposure to Dioxin for Long Periods Can Boost Cancer Risk."
- Washington Post story by Malcolm Gladwell — "Extensive Study Finds Reduced Dioxin Danger."
A later commentary says:Gladwell says he chose not to quote the scientist who wrote the editorial because he didn't know whether the author's views represented a scientific consensus. Instead, he quoted George Carlo, whom he identified as "one of the nation's leading dioxin experts."
At the time, Carlo had not published a paper on dioxin in a peer-reviewed journal, a publication that only publishes papers reviewed by scientists in the same field.
More important, Carlo is a specialist in risk assessment and management whose clients then included the Chlorine Institute, a trade group, and Dow Chemical — both affected by dioxin regulations. Gladwell did not cite Carlo's industry connections. No one else was quoted.

1991 Jan 17: & 30 The Agent Orange Act of 1991 passed the House (412—0) on the 29th and the Senate (99—0) on the 30th. This put the National Academy of Sciences (NAS) in control.
1991 Jan 23: Four CEOs of paper companies as representatives of the American Paper Institute visited EPA Administrator William Reilly. They later wrote thanking him:
'We were also encouraged by what we perceived as your willingness to have the agency move expeditiously to re-examine the potency of dioxin and chloroform in light of the important new information that has been submitted with respect to those chemicals,' [truncated][Source: The Dioxin Wars, downloadble on-line]
[There was now a] 'prevailing view that low-level dioxin exposures do not pose a serious health threat' [and] 'despite this new reality, EPA has taken no tangible or timely steps to revisit its health criteria for dioxin, and has even failed to temper the agency's zeal in acting on the worst risk estimates... '.
1991 Jan 29: Banbury Center Conference fiasco
Ellen S Ebert of ChemRisk has written to Robert K Sells of Bob Sells PR Associates in Little Rock Arkansas on behalf of Dr Keenan of ChemRisk.
[This is the Dr Keenan who organised the Kociba study re-evaluation — thus creating the misleading impression that dioxin was not so dangerous. See the abstract of the re-evaluation article, now commonly ridiculed. 
Elbert writes "... at Dr Keenan's request I am enclosing the following materials pertaining to the Banbury Conference" [Oct 22-24 1990]
The Banbury Report pdf file: has been subdivided into; Part 1 Part 2 Part 3
- Carlo Report
[At this time Carlo was with SUNY at Buffalo School of Medicine, and simultaneously chairman of the HES Group, Washington DC.]- [Linda] Birnbaum Memorandum [EPA]
- Summary by [C.A] van der Heijden [Nat. Inst. of Public Health and Environmental Hygiene in Netherlands]
- Summary by [Robert] Scheuplein [of FDA]
- Summary by [Michael A] Gallo [Robert Wood Johnson Med School, NJ]
- Chlorine Institute Press Release (Draft)
[Prepared Nov 1 1990 by George Carlo for The Chlorine Institute.]
This long file makes a few key points:
- Carlo's public-release report questions the value of using animal studies to assess human risk from dioxin exposure and says "scientific evidence strongly suggests that dioxin at very low levels does not constitute a significant health risk to humans.
- Carlo's Chlorine Institute report, "Biological Basis for Risk Assessment of Dioxins and Related Compounds."
[Page 1 of this report is missing] It apparently dealt with establishing that dioxins have a non-linear response, because it concludes that there are safe levels of exposure.During the conference, it was noted that no one presented a scientifically sound rationale for use of the linearized models for accurately estimating cancer risks for dioxin.
There was support among the scientists for the Margin of Safety (MOS) approach to assessing dioxin's risk which is consistent with the concept of a practical threshold for adverse effects.
[It discounts the original Kociba findings and says] "that the incidence of liver tumors in that study is less than reported originally in 1978, when today's pathologiy classification standards are applied. - Dr Vernon Houk of the CDC expressed a desire to revisit the dioxin risk assessment position held by that agency.
Dr Scheuplein of the FDA noted that his agency would also consider a change in position based on the new data.
Dr Farland of the EPA was less committal. - Report from Linda Birnbaum of the FDA [Note page 2 is missing] She sums up the situation at this time as:
"Dioxin clearly has the potential to cause serious health effects. However, exposures, except in the few accidental poisoning episodes, have been low. As long as exposures do not increase, dioxin should not pose a health threat.
- The other three reports were not found in this file.[Not from the tobacco industry]
1991 Feb 22: Banbury Center Conference fiasco
Science magazine publishes "Flap Erupts Over Dioxin Meeting," an article about the Banbury Conference fiasco. It expressed the outrage of Ellen Silbergeld at the misrepresentation of her views and those of others at the conference. The article discussed the...
"furor that erupted in early February when participants learned that a public relations firm hired by the Chlorine Institute, which helped pay for the conference, was circulating a "consensus" summary of the meeting"[... which essentially said dioxin dangers had been grossly overstated. In fact, this 'consensus' had only been tentatively considered and then rejected by many of those who participated.]
The director of the Banbury Center, Jan Witkowski, then wrote a letter of apology to the meeeting participants and to the press, disassociating himself and his organization from the fiasco. Even Vernon Houk said that he was "disturbed" by the publicity campaign saying; "I don't think it is fair to represent consensus when none was really sought."
However they don't blame the scientists who organised and ran the meeting. The article lays the blame for the fiasco squarely on Carlo:
The three organisers picked all participants, with one exception — George Carlo — who was invited as an observer for the Institute at its request. Carlo, an epidemiologist and lawyer who heads the HES Group in Washington DC, is a regular consultant to the Institute.Edelman had sent out the press packet which included statements from the three chairman along with a background paper written by Carlo that none of the organizers or participants had seen.
None of the participants Science spoke with, nor even meeting co-organiser [Michael] Gallo, knew Carlo was there as the Institute's observer.
They also assumed that Edelman [the PR Company] was representing the Banbury Center, not the Chlorine Institute.
See later pages in this package of documents: 

that applauded me for taking on the Chlorine Industry as they used my name on a report that I did not write, and I called them on it very publicly. |
See the actual Science article with no applause. 

1991 March 1: Lawyer James Baller, George Carlo, and his secretary Kelly Sund have jointly written. "The Agency for Toxic Substances and Disease Registry — A Growing Power in the Hazardous Waste Arena."
This is a detailed history of ATSDR (The Superfund agency) with a so-called 'critical examination of its activities'. However Carlo and Baller were also collaborating with the ATSDR in preparing promotional material distributed via the Washington Legal Foundation (WLF).
1991 Mar 12: An op-ed, "Thumbs on the scales of Risk?" by George Carlo and Kelly Sund, is published by the Washington Times. The op-ed's byline claims that Sund [Carlo's secretary] "specialises in risk assessment."
It then quotes Beveridge & Diamond's top lobbyist for the chemical industry (ex-EPA director) William Ruckelshaus on the need for risk assessment to be "based on scientific evidence and consensus only". [presumably, he also favours motherhood and apple pie.] These views are apparently supported by Berkeley biochemist, TASSC advisory board member and tobacco industry friend, Professor Bruce Ames — who is also credited as an "authority on risk assessment."
The slant of the op-ed is to attack the EPA on its classification of ETS and other harmful substances like dioxins and some pesticides, by suggesting that these assessments are politically motivated.
[Note, however, that no one now seriously contends that "Alar... radon...dioxin" are not all genuine reasons for concern. However the corporate scientific lobbyists claimed that these were all examples of failed doomsaying predictions. They were trotted out to support corporate claims that scientific bias and "fear generated by worst-case risk-assessment" were the real problems. Of course, over-zealous activists also provided the corporation lobbyists with some good arguments.]
This Carlo/HES op-ed in the Washington Times is a thinly veiled attack on the EPA's ETS Science Advisory Board, and it conforms to the requirements set out in the Philip Morris plan of November 10th. Carlo wrote about his Bias study:
A recent survey by our firm illustrates how "a value-laden process'' can warp sound, objective science.The solution Carlo and Sund offer is for governments to mandate standards for risk assessment to be used by all the regulatory agencies. [This would then elevate the role of economists with their cost-benefit calculations to a position above any biomedical expert's judgement.]
We asked 1,461 US scientists questions regarding the health hazards of three substances associated with current environmental health issues: environmental dioxin, environmental radon and environmental tobacco smoke. The purpose was to describe the interaction between science and preconceived judgments that affect policy.
For dioxin and radon, knowing the name of the substance made little difference in perceptions of risks when respondents were read a series of facts. Yet with environmental tobacco smoke, simply telling them the name of the substance produced radical swings in their assessment of risk.
According to OMB, there have been overestimates of the risks of dioxin to humans in the environment by as much as 5,000 times or more, which may have cost the public billions of dollars in direct and indirect expenditures.
[Here we have an epidemiologist quoting the White House Office of Management and Budget as an authority on the possible health risks of dioxins!]
Dr Vernon Houk, the director of Environmental Health for the Centers for Disease Control, has said, "The general public thinks these risk estimates are based on real science, but that simply isn't true."
The article then moralises in ways that will tug your heart-strings:
The American public deserves better than to have to wait 25 years, watching billions of dollars of public and private money expended and significant portions of American agriculture and business destroyed.[By a happy coincidence, projects aimed at introducing mandatory risk assessment into all of the federal regulatory agencies were being conducted at this time by both the chemical industry and the tobacco companies.]
The risks to our society must be assessed, regulated and communicated. Most of us want to save the planet, the children, the dolphins. Few of us know how. But members of the scientific community are developing new methods incorporating real-world assumptions, and that is a good start.
We can fix the risk assessment problem. It's up to science — good science, unbiased science — to establish a real-world standard and the real-world studies to meet that standard. It is up to responsible journalists to report accurately, clearly, without bias or hysteria. And it is up to the public to demand and to the government to implement, not merely study, regulatory risk assessment reform.


[All they needed as background to this stirring message was Federal Focus's own jazz band playing the Star Spangled Banner. ]
Note that the Washington Times is the newspaper of choice for lobbyists. It was run by the Rev Sun Myung Moon and his Unification/Moonie cult, which also had its own UPI press agency for syndication.]
1991 March 22: Banbury Center Conference fiasco
The American Legislative Exchange Council (ALEC) newsletter says that experts were now discounting the dangers of dioxins because of a new epidemiological study of chemical industry workers.
[ALEC was totally funded by manufacturing companies as a way to get access to compliant State and Federal politicians]
Remarked Dr George Carlo, chairman of the Health & Environmental Sciences Group and one of the nation's leading dioxin experts,"This is very reassuring. Here we have the group of people who have been exposed to higher levels of dioxin than perhaps anyone in the world, followed for a longer period of time than any other study, and it is not bearing out the cancer risk hypothesis. If we were going to see something, we would see it here in spades. And we haven't.
Given the exorbitant costs of dioxin control regulations, state legislators would be well advised to review their own stale standards and modify them accordingly.


1991 Mar 26: Burson Marsteller has set up a press briefing to counter the release of the EPA ETS Risk Assessment categorising second-hand smoke as a Class A carcinogen. They have sent this draft press release to Philip Morris for approval.
Two old lags who had long worked for the tobacco industry, Paul Switzer of Stanford University and Joseph Fleiss of Columbia University, were to present their conclusions about the poor quality of the EPA's work. They were prepared to say:
[Carlo later changed his mind and refused to be interviewed on-the-record either.]"That the scientific evidence is conflicting and does not support such conclusions."
Because of the hostility to any views, no matter how authoritative, which contradict anti-tobacco sentiment, the two eminent scientists are prepared to speak only on the condition that they not be publicly identified.
In Addition: Dr George Carlo is prepared to detail the results of a study conducted by his firm that indicates such personal bias against tobacco in the scientific community that the nature of the attacks becomes immediately understandable and any results highly questionable.
The scientific presentations will be buttressed by Steve Parrish, General Counsel of Philip Morris, who will describe the scientific and medi,a environment which has produced the attacks - outlining significant tobacco industry mistakes in dealing with it - will acknowledge the current social climate against smoking and will describe work by his company which points toward solutions.
This presentation will be made only to media columnists willing to agree to protect the identities of the two aforementioned scientists [Switzer and Fleiss] starting with Peter Huber, Warren Brooks and Fred Barnes.
• Huber was the 'junk-science' consultant at the Manhattan Institute who wrote a column in The American Spectator
• Brooks and Barnes were far right-wing columnists and tobacco industry supporters.
If those columns could be obtained, and that is by no means certain, then other media opportunities may suggest themselves, but otherwise the prognosis for media support or even balance must be regarded as bleak.


1991 Apr: - May Philip Morris made a number of payment to Thorne Auchter for services related to the EPA and OSHA. [Auchter was also a silent partner in George Carlo's science for sale operation Health and Environmental Sciences (HES)]
Immediately following in Philip Morris's accounts are payments to Carlo's HES of:
- Apr — $ 1,728.37 + $1,668.20 + $4,756.80 + 1,931.50
- May — $1,460.50
[These appear to be monthly consultancy time-charges. They are each coupled with what appears to be Washington-to-Dulles two-way cab charges.
- Jan — $4,435.00 + $ 86.95 expenses
- Mar — $5,600.00 + $67.40 exp
- Apr — $5,600.00 + $67.40 exp
— $8,840.00 + $65.65 exp- May — $8,840.00 + $65.65 exp
- June — $13,019.00 + $36.00
— $6,320.00 +$150.20 exp- Aug — $12,707.50 + $182.16 exp
- Oct — $6,671.50 + $175.93 exp
They are probably expense claims for Carlo's travels to talk to the media ( Philip Morris would have set up his schedule and paid the airfares direct.)]
1991 Apr 18: The EPA issued a draft of the ETS Risk Management paper which signalled their intention to classify passive smoke as a Class A [known human] Carcinogen.
1991 May: ('spring') Environmental Claims Journal [insurance industry] publishes a paper by Carlo, "New Science in Risk Assessments." It is another attempt to extract the maximum from of the 'Health Scientists Survey' to attack the EPA:
In a 1990 survey of over 1,400 American public health scientists from government, industry and academia, more than 90 percent of the respondents indicated that they believe public health funds in the US are being misdirected by the government.


[The "over 1,400" scientists shrunk to "nearly 1,300" by the time the study was published a year later.
This article was widely cited by tobacco industry scientists in their own papers.]
1991 May: At a conference sponsored by Syntex (the company largely responsible for polluting Times Beach), Vernon Houk was interviewed by a reporter with the St Louis Post Dispatch as saying that he had made a mistake in urging the federal government to evacuate Times Beach, and that "many scientists now believe dioxin isn't as bad as we thought." He was then quoted in a front-page New York Times article by Keith Schneider.
It later transpired that Houk's CDC proposal for relaxing dioxin standards was copied word-for-word from reports given to him by the paper industry. [Waste Not, 1991]
[Keith Schneider attributed to some 'expert' his own made-up statement that exposure to dioxin was "no more harmful than a week of sunbathing". Twenty major newspapers then paraphrased the NY Times article, an used Schneider's fictitious statement to lambast scientists for sowing panic.]
| Times Beach |
|---|
| The major cause of this famous dioxin problem appears to have been contractor Russell Bliss with his "dust-binding mix 'of waste oil from gas stations and Agent Orange residue, and other extremely toxic, highly dioxin-contaminated waste from companies in the chlorine chemical industry (Syntex, Hoffman-Taff, Nepacco, Independent Petrochemical Corporation, IPC, and Charter Comp).
This stuff was sprayed on all dusty surfaces in the eastern half of Missouri, including all city streets in Times Beach, numerous trailer parks, truck terminals, a large pumpkin grounds, his own farm, and virtually any of the many horse racing tracks in eastern Missouri. Over a short space of time 75 horses and several cats and dogs died — and then the CDC's Vernon Houk ordered the permanent evacuation of 2000 residents. |
1991 May: Three years before he retired through the revolving door, Dr Vernon Houk, Director of the Center for Environmental Health and Injury Control, back-tracked and announced that he believed dioxin was only "a weak carcinogen'. Houk had been the authority behind the Times Beach scare.
Houk's statements formed the "news hook" that allowed [Malcolm Gladwell of] the New York Times to climb on board with its own page-one story August 15, 1991: "U.S. Officials Say Dangers of Dioxin Were Exaggerated."
With the Post, the Times, Houk and Reilly all speaking with one voice, the "detoxify dioxin" campaign was clearly succeeding.

1991 May 15: Payments made to the chemical industry's defence witnesses by Syntex, IPC and Nepacco in Times Beach dioxin cases.
This comes from the evidence of Dr Gerson Smoger, the lawyer of a large number of Times Beach dioxin victims
[Carlo has occasionally claimed that he worked FOR the Times Beach VICTIMS]
| Witness payments | |||
|---|---|---|---|
| Name | Est. | Paid | Rate/Total |
| D.Bissel — Berkley CA | $ 140 p.d | $ 8,400.00 | $ 10,920.00 |
| H.Busch — Houston TX | $ 90,250.00 | ******* | ******** |
| G.Carlo — Wash. DC | ******** | ******** | $ 21,154.85 |
| R.Harbison — Tallah. FL | ******** | ******** | $ 9,080.31 |
| J.Harris — Chicago IL | $ 16,850.00 | $ 32,550.00 | $ 350 p.d |
| E.Johnson — Durham NC | $ 33,600.00 | $ 42,000.00 | $ 300 p.d |
| S.Lamm — Wash. DC | ******** | ******** | $ 34,363.75 |
| S.Sternberg — New York NY | $ 14,688.00 | $ 25,488.00 | $ 300 p.d |
| C.Tamburro — Louisville KY | $ 10,527.50 | $ 18,527.00 | $ 350 p.d |
| S.Tucker — Houston TX | $ 6,125.00 | $ 11,375.00 | $ 500 p.d |
| J.Bunnet — Santa Cruz CA | $ 2.300 p.d | $ 8,855.00 | $ 21,270.00 |
| L.Hov — Sacramento CA | $ 11,757.67 | $ 26,000.00 | $ 1.500 p.d |
| T.Tierman — Dayton OH | $ 1.000 p.d | $ 9,281.25 | $ 13,531.25 |
| M.Zabik — Williamstown MI | $ 16,500.00 | $ 9,000.00 | $ 1.500 p.d |
| TOTAL $ 373,010.66 ** | |||
| * The total revenue from Busch, Carlo, Harbison and Lamm are unknown. For the overall calculation, those details before 4/15/91 have been used. ** The total represents a very conservative estimate. | |||
and consumer products, as well as the safety and efficacy of pharmaceuticals and medical devices. |
1991 May 28: Tobacco lawyers Shook Hardy & Bacon are billing Tom Borelli at Philip Morris for work done quite independent of their work for the Tobacco Institute. They are charging PM $1,753.33 for reading and giving legal clearance on...
... an ETS manuscript, ETS Fact Sheets, and the Carlo paper.


1991 June 6: Carlo's OTA/dioxin associate Michael Gough is the keynote speaker at a Washington luncheon mounted by the National Chamber Foundation (the lobby arm of the US Chambers of Commerce) on "Dioxins and the Risk Assessment Process."
Philip Morris's representative [Mayada Logue] comments.
Michael Gough from the Office of Technology Assessment (OTA), the research arm of Congress, will be talking about dioxin and the risk assessment process.
Gough was later quoted in a newspaper article [Washington Times 15 Apr 91 - "EPA to rule on risk of Secondhand Smoke"] as saying: "I am not convinced that (secondhand tobacco smoke) is responsible for anything more than an increased occurrence of colds in children".


[This actually was a complete reversal of Gough's position in Oct 1990. He had once asked Tom Borelli to take him off the Philip Morris mailing list because he was opposed to smoking. He was now about to leave the OTA and looking for consulting work.]
1991 June 20: The New England Journal of Medicine (NEJM) has published a letter from George Carlo, Kelly Sund, et al (HESG) which appears to take a contrary position to JC Bailar III on "Dioxins and mortality from cancer" It makes the claim that
Respiratory cancer is excessively difficult to link to dioxin because the researchers are unable to control for cigarette smoking and occupational exposure to other substances.The Address given for Carlo's Health & Environmental Sciences Group (HESG) is now Washington DC/Canada.


[This overnight development of a Canadian address may mark the beginning of a formal relationship with Ian Munro of Cantox.]
1991 Jul: /E Philip Morris is compiling a two-part book to attack the EPA's Risk Assessment of ETS,
The purpose of Part I of this book is to provide a clear, concise picture of the science on ETS and the EPA's risk assessment process.Confidential quotes were being collected along with useful data. One section describes Carlo's bias study which was still nearly 9 months from completion (Mar 1992)
Part II provides an overview of the status of indoor air quality research and the role of ventilation in addressing the true causes of the problem, often wrongly attributed to ETS.
The Health and Environmental Sciences Group, an independent scientific consulting firm, conducted a survey of 1,461 US scientists to determine whether there were preconceptions among scientists on risks related to certain substances undergoing risk assessments by EPA. (See Appendix ).The responses followed in a table... plus the comment:
[Leaving space for the Appendix number, when it was finally written.]Based on the information, the scientists were asked to estimate the degree of environmental hazard and perceived health risk posed by the substances.
- Half the scientists were given brief descriptions of the available scientific data on three substances, identified only as x,y and z.
- Half of the scientists were given the same brief descriptions; however, the substances were identified: dioxin, radon and ETS.
(This entire section will be held until publication of Carlo survey)


[Life is so much less complicated when you know the results of your 'bias' survey 9 months before the study is complete.]
1991 July 31: Maureen Jablinske, Research Associate with Carlo's Health & Environmental Sciences Group, has written to Tom Borelli at Philip Morris, sending him risk tables and saying:
Here are additional tables which include example of relative risks less than 2.0, deemed insignificant from a public health perspective.
George would like to discuss this and other relevant matters with you in the near future.


[Borelli was looking for examples of 'junk-science" which could be blamed on the EPA's use of low relative risk figures (which are never used by regulators in isolation). Carlo was clearly doing literature research to provide them with ammunition to attack the EPA.
The idea that any study with a relative-risk figure below 2 could be ignored, was a dream that the tobacco executives pursued endlessly. It was the main driving force behind their attempts to reformulate Good Epidemiological Practices (GEP)]
1991 Aug 15: Banbury Center Conference fiasco
The New York Times quoted both the CDC's Vernon Houk and the EPA Director Reilly — both of whom believed chemical industry lobbying claims that the scientists had reached a consensus agreement on dioxins at the Banbury Center meeting.
The editorial and two front page stories all downplayed the hazard of dioxins:
One article headlined 'U.S. Officials Say Dangers of Dioxin Were Exaggerated' stated that "Exposure to the chemical, once thought to be much more hazardous than chain smoking, is now considered by some experts to be no more risky than spending a week sunbathing."Thirty other newspapers carried these stories, as did Time, Los Angeles Times and Chicago Tribune.[This was months after Science magazine had exposed the Banbury fiasco.]
The other article called for relaxation of "the current strict and costly standards" for dioxin.
No journalists bothered to contact the CDC to see what they thought of Houk's claim that the CDC had made a mistake in evacuating Times Beach. Twenty years later the CDC maintains that they would have taken the same action, based on the most up-to-date scientific evidence.

1991 Sep 21-22: The International Congress on Dioxin was held at Chapel Hill, North Carolina
1991 Sep 23: Banbury Center Conference follow-up
The International Congress on Dioxin, held at Chapel Hill, North Carolina became the subject of an expose of the lack of intelligent balance exhibited by National Public Radio.
[NPR's] Morning Edition host Bob Edwards announced that scientists were gathering in North Carolina to discuss recent studies suggesting that "the dangers of dioxin may be overrated."
NPR science reporter Richard Harris led off with interviews with two government scientists, Michael Gough of Congress's Office of Technology Assessment and Linda Birnbaum from the Environmental Protection Agency. Both suggested that new studies might lower estimates of dioxin's danger; Gough was quoted saying that the risk of cancer from dioxin "may be zero."
[He later claimed you could eat a plateful of breakfast cereal soaked with dioxin instead of milk!]
These remarks were countered by those of public interest activists: Ellen Silbergeld, a toxicologist identified as working for the Environmental Defence Fund, and Paul Connett, an "anti-incinerator activist."
[Incinerators also produce dioxins.]
Harris also cited an unnamed federal official [Vernon Houk] who had ordered the dioxin-related evacuation of Times Beach, Mo., who now says the evacuation was unnecessary.
Harvard Center for Risk Analysis (HCRA)
[Carlo and Graham are closely associated in servicing both the tobacco and the cellphone industry from 1992-3 on.]
1991 Oct 21: John Graham at the Harvard Center for Risk Analysis [which is not a part of the University or the School of Public Health] has written to David Greenberg at Philip Morris promoting his organization and seeking a donation.
He wants a face-to-face meeting "to learn more about the risk-related challenges that you face" and $25,000 for each of the following two years to "help the Center expand its public policy activities." He boasts that:
The Center has been launched primarily with gifts from the following corporations: the Amoco Company, Bethlehem Steel Corporation, British Petroleum, Chevron Corporation, The Coca Cola Company, Dow Chemical Company, Eastman Kodak Company, Exxon Corporation, General Electric Corporation, General Motors, lnland Steel Industries, Merck & Company; Mobil Oil Corporation, the Monsanto Company; Pepsico Incorporated, Rohm and Haas Company, Texaco, Union Carbide Corporation, and Westinghouse Corporation.
Government support has also been provided by the Centers for Disease Control, the US Department of Transportation, and the National Science Foundation. The Center is now booking to a broader base of industrial sources to supply critical funding for the ycars ahead


1991 Oct 28: Philip Morris USA now has a detailed plan for attacking the EPA and OSHA and other regulators. It allocates specific tasks to its main disinformation executives:
- TB = Tom Borelli who runs Scientific Issues/S&T in the USA
- HGA = Helmut Gaisch (who runs the Scientific Issues/S&T in Europe)
- JB = James Bowling, special assistant to the CEO
- MP = Mary Pottoroff, major project manager
- RP = Robert Page, manager Scientific Issues
- ML = Mayada Logue, assistant Scientific Issues
- SCP = Steve Parrish, Vice President, Corporate Scientific Affairs
- TI = Tobacco Institute
- CORP.AFF = PM International Corporate Affairs under Andrew Whist
Establish a coalition of business groups and others to reform the risk assessment orocess of the federal government
— Executive Order
— White House office of Science and Technology
— )oin the White House committeee on Environmental issues
— Publication/presentation of risk assessment papers ; Carlo Studies


1991 Oct 29: Robert Page of Philip Morris is commenting to his superior Steve Parrish on the begging letter from John Graham (Oct 21)
My reaction: I think that a meeting should be arranged with Graham when he calls [David] Greenberg [PM Washington] and that you should try to attend. It doesn't hurt to talk to the guy — he says: "It is important for me to learn more about the risk-related challenges.that you (PM) face."
Why not take him up on the offer? Sure, he's after $ to help support his Center, but whether or not PM decides to contribute, it's more important to meet him and perhaps get 'looped in' better with his activities.
From all that Mayada [Logue] has learned, he is a key player in all this risk analysis stuff that's currently going on in the government. (Depending on the 'vibes' you guys get when you meet Graham, I would also be in favor of PM becoming a contributor to the Center.)


[At this stage Philip Morris was as cynical as you'd expect about Graham's promotional attempts with the implication that he could offer special services on ETS. Later they became one of his most generous supporters.]
1991 Nov: The Journal of Toxicology and Environmental Health published the Dow Chemical re-evaluation of Richard Kociba's original findings that dioxins were producing cancer in female rats. [The Squires/Chemrisk study]
The chronic bioassay of [dioxins] reported in 1978 by Kociba et al. has been considered to be the primary evidence supporting its carcinogenicity, and is the basis for most dioxin regulations in North America and Western Europe.
Because the histopathological criteria for proliferative lesions in the rat liver have changed significantly since 1978, a reevaluation of the liver slides was conducted recently by an independent panel of pathologists. Using current National Toxicology Program criteria, their study showed, in contrast to the original findings, that about two-thirds fewer tumors were present in the livers of female Sprague-Dawley rats.
These results indicate that the carcinogenic risk to humans from exposure to 2,3,7,8-TCDD is at least 16-fold lower than previous estimates derived from the Kociba et al. (1978) bioassay.
This article, now commonly ridiculed, was by RE Kennan, Dennis J Paustenbach, RJ Wenning, and AH Parsons, of ChemRisk (aka McLaren/Hart of which Paustenbach was President)

[Squire's name isn't on this study even though he supposedly supervised it.
See also a second dioxin reassessment done by Paustenback, John Doull, John D Graham, Hanspeter Wisch and Michael Gough in 1995. (all tobacco lobbyists also)]


1991 Nov 6: The Nufarm dioxin spill: Greenpeace in Australia published "Media lies and the new McCarthyism" The organization was under attack for its 'scare-mongering' about the dangers of the Antarctic ozone hole. Also:
The present cause cèlébre of the green-bashers is NuFarm, a Melbourne-based chemical company forced to close temporarily and submit to Australia's first environmental audit, at a cost to it of $5-6 million, following a spectacular Greenpeace action in 1990.
Greenpeace had discovered dioxins and furans in the company's discharges into Melbourne's sewer system, with the possibility these could find their way into the food chain via meat from animals raised at the Werribee sewerage farm.
The company had been warned previously over its waste-dumping practices, and is now facing revocation of its licence to discharge chemical waste into the sewers because of its refusal to sign an agreement with Melbourne Water. Unless it signs the agreement or finds another method of waste disposal, it could be forced to close by early 1992.
The 1990 [Victorian government] audit eventually found that the company was not discharging dangerous levels of dioxins or furans, a finding that Greenpeace disputes, claiming there is scientific disagreement. Some scientists now say that dioxin is less dangerous to humans than previously thought, a dramatic departure from the previous acceptance that it is one of the most toxic chemicals known.
As a result of the Nufarm episode, the group has been widely accused of using the tactic of the big lie. Whether or not Greenpeace was right in all details of its claims against Nufarm, the real big liars of our time are those who seek to conceal and underestimate the extent of the environmental and social problems facing our planet.

1991 Dec: [Carlo claim]
As Chairman of Health & Environmental Science Group, Carlo headed a pesticide industry task force to evaluate the relationship between pesticides and cancer.On closer examination however this claim turns out to be a reference to:
In December 1991. an independent scientific review panel was convened hy Drs. Ian Munro and George Carlo to evaluate critically the methodology and findings of a study by Hayes et al. (1991). "This independent peer review was supported hy the Industry Task Force II on 2,4-D Research Data.
[Warning: This is a very large file]


1991 Dec: The HES team has completed the ['Bias Study') "Health Scientists Survey: Identifying Consensus on Assessing Human Health Risk" for Philip Morris. The HES group consists of George Carlo, Sydney D Pettygrove, Kelly Sund, Maureen Jablinske, and Nora L Lee. They have come to the pre-ordained conclusion that:
- Weight of evidence approach is preferred
- Corroboration between animal studies and human studies important in identifying public health risk
- Decisions about human health risk based solely on animal models are suspect
- Strong belief that the public does not understand public health risk information
- Strong belief that public health dollars in the United States are not targeted properly to reduce environmental health risks


The study was to be published in Environmental International, July/Aug 1992 as another critique of the supposed bias of scientists. It does not mention that Philip Morris was the source of its funding [and therefore possibly a source of bias in their findings] and maintains:
Ackowledgment — This survey was conducted during 1990 and 1991 with the support of the Institute for Regulatory Policy.[Thorne Auchter's think-tank][The study was used in a 1993 courtcase in defence of RJ Reynolds.]


1991 Dec: This is a tobacco industry power-point lecture slides for Carlo's Health Scientist Survey. The slides show some data which is contrary to some of their later claims (they 'adjusted' some of the figures):
- Note that: 63.7% of the expert scientific respondents agreed that a Risk Ratio of 1.3 in animal studies could be interpreted as demonstrating "Probably Health Risk"
Only 11.4% thought it showed "No Health Risk."
Despite this finding, they continued to make these claims.]


In the process it became evident that it was also possible to skew a study in order to produce a pre-ordained outcome. |
1991 Dec: Tobacco lawyers Shook Hardy & Bacon report on Carlo's Health Scientist study:
A recent survey of human health scientists reportedly suggests that many scientists feel that federal funds spent for reducing environmental health risks are improperly targeted.
The December 1991 survey was conducted by Dr George Carlo, chairman of the Health and Environmental Sciences Group, a Washington-based scientific consulting firm specializing in regulatory issues, and was commissioned by the Institute for Regulatory Policy, a regulatory reform group headed by former OSHA chief Thome Auchter.
Given to 1,292 health professionals in epidemiology, toxicology, medicine and other disciplines, the survey reportedly suggested that 81 percent of the respondents believe that "public health dollars for reduction of environmental health risks in the UlS. are improperly targeted." Eighty-seven percent of the respondents reportedly found it impossible to calculate human cancer deaths based solely on animal data.


[Shook Hardy & Bacon would have helped set up the Institute of Regulatory Policy for Philip Morris, and they certainly knew who operated it, and why it was being funded... as did George Carlo.]
- Structural diagrams of the Corporate Scientific Affairs division and staff of the (This is after the Nov 1989 restructure. It is now under the control of lawyer Stephen C Parrish (who was also PM's Special Counsel).
- Also see $300,000 check to be paid to Covington & Burling (June/Aug 1991) as a supplementary grant for the Asian ETS Consultants Program [Whitecoats recruitment] followed by another $10,000 for some EPA work
- C&B was laundering payments of $440,111 to the Asian WhiteCoats for the first half of 1991 (with $71k for C&B's expenses, etc) and were currently carrying nearly half-a-million dollars owed by the tobacco industry. The overall budget for the project had been originally been set at $1.1 million for the recruitment of 12 to 15 WhiteCoats. It had since expanded.
- PM Corporate Scientific Affairs contract with Burson Marsteller (PR). B-M's billing rates (for different states) extended from $350 per hour down to $50 per hour, with most client service work in the $200 p.h. range. They were paid roughly $40,000 (+ expenses) per month by this division of Philip Morris.
- Barrera Associates: Lisa Barrera's EPA surveillance operation is also contracted here, with monthly payments of $20k. She had insider contacts in the agency who leaked information.
- Articles of Association and Bylaws for the Center for Indoor Air Research (CIAR). Philip Morris had instigated this scientific front organization and C&B had prepared the incorporation documents. Philip Morris had put in $3.1 million, RJ Reynolds $2.2 m, and Lorillard $0.6 m
- The Business Council on the Reduction of Paperwork, a corporate front for continued attacks on the regulating agencies (which changed its name regularly), was to receive a "Grant-in-aid' of $300,000 from Philip Morris.
- Federal Focus Inc. (run by Carlo's partners/friends Jim Tozzi and Thorne Auchter) was also to receive a "Grant-in-aid" of $300,000. These two payment were handled by Amy Millman and made in two checks to be hand delivered. [Federal Focus would normally have been paid via MBS direct.]
- Paperwork on a dispute about the accounting allocation of $8.7 million in costs from PM's European (FTR) Science & Technology division in Neuchatel, Switzerland: 63% is to be allocated to Corporate Scientific Affairs (the US disinformation division).
The job description of this Swiss FTR unit was now:- Providing strategic input on proactive measures regarding ETS and IAQ;
- providing Corporate Affairs personnel with scientific support with respect to ETS and IAQ issues;
- providing advisory and spokesperson services regarding ETS and IAQ in contacts with scientists, journalists, politicians and various institutions;
- acting as a liaison between Corporate Affairs personnel and Covington & Burling regarding the consultants program [WhiteCoats];
- monitoring the scientific literature regarding ETS;
- providing Corporate Affairs personnel with scientific support regarding specific issues such as health warnings, regulatory issues and the efforts of various third-party institutions, and;
- advising senior management on matters relating to ETS and IAQ.
- Tom Borelli (S&T in the USA) has an accounting for his own division which includes budgets and expenditure deals under the headings:
- Staff expenses
- Seminars and Workshops
- Scientist consultants (Rylander, Tso)
- Contributions (National Journalism Center)
- Consultants (all propaganda producers)
- Hines & Quinn $100,000 (Setting up a journalist network)
- Burson Marsteller - $0.5 m (PR)
- K Cleveland $25,000 [This is syndicated columnist Kathleen Parker who publicised their claims that passive smoking had no effect on children.]
- George Carlo $25,000 (HES operations)


1992 Jan 21: Transcript of Legislative Assembly of Ontario, Standing Committeee on Social Development, hearings on the Waste Management Act, 1992 George Carlo's appearance was as a witness at the request of the St Lawrence Cement Co. of Mississauga.
Dr George Carlo is here. Dr Carlo is chairman of the Health and Environmental Scientists [sic] group in Washington, DC.[Through these very subtle choice of words Carlo's company HES has been made to appear as if it were a genuine scientific association, with him as the chairman of some independent review board.
[Note: 'chairman' of the 'scientists group']
He is here as an independent adviser. He is chairman of the scientific advisory board reviewing use of waste fuels in cement kilns in the United States, particularly the health and safety aspects associated with that.
[Again, the misleading inference that his evidence is as an 'independent' head of a review board.']
Dr Carlo can answer any questions and would be available at any time to the committeee.
Cement kilns were incinerating waste material to generate heat — and when hazardous waste was used (old oils and tiers) they produced dioxins. Carlo had a contract with the US cement/incinerator/waste group.]

1992 Jan 23: Tom Borelli is flying to Washington to meet with Richard T Hines to discuss their work in support of Philip Morris's ETS projects.
[Hines & Quinn — also associated with the Southern Confederacy and the CIA.
They ran an op-ed writing bureau and had links to the (unfettered free-market) National Journalism Center — and were attempting to set up a similar operation in Eastern Europe with Philip Morris money, via the Atlas Network.]
Borelli's trip was cancelled due to weather. But he met with Burson-Marsteller and Joseph Wu on the 28th.
[Wu was the tame scientist from NY Medical College who Carlo hd helped recruit in 1989]
This document (Page 161) records that the budget for...
Borelli's Science & Technology division of PM (via Borelli) also:
- Gives $25,000 to the National Journalism Center.
- Gives $25,000 to Joseph Wu's New York Medical College.
- Employs Hines & Quinn as consultants for $100,000.
- Employs "Carlo" as a consultant for $25,000.
[This would be a Carlo consultation fee, not HES project work.]
1992 Jan 24: The Philip Morris/APCO controlled EPA Watch newsletter has a leaked preview of Carlo's "Health Scientists Survey".
[Environmental International, the journal which published it, only received their copy of the paper March 13.]
Survey says scientists feel US funds for reducing health risk misdirected.
Most human health scientists say that federal funds spent for reducing environmental health risks are improperly targeted, according to a new survey condiicted by an independent science group. The findings suggest the need for overhauling the way the federal government makes environmental risk decisions, its sponsors say.
Some scientists warn, however, that the survey questions were asked in isolation, and did not reflect "real world" or case study scenarios.
[A very clever attempt at being seen as an even-handed commentator.]
The December 1991 survey was conducted by Dr George Carlo, chairman of the Health & Environmental Sciences Group, a Washington-based scientific consulting firm specializing in regulatory issues, and was commissioned by the Institute for Regulatory Policy (IRP), a regulatory reform group headed by former Occupational Safety & Health Administration chief Thorne Auchter
In the survey, 1292 health professionals in the field of epidemiology, toxicology, medicine and other disciplines were interviewed.
Sources associated with the project say they plan to present the findings to EPA's Science Advisory Board staff, and share the survey results with members of Congress.
Key findings of the survey show that
- 81% of the respondents believe that "public health dollars for reduction of environmental health risks in the US are improperly targeted."
- 87% responded that it is impossible to calculate human cancer deaths based solely on animal data, with
- the data from the survey "supporting a better method for assessing environmental health hazards, a 'weight of evidence' approach that takes all plausible human and animal data into account,"
- according to documents accompanying the survey, IRP sources say the survey shows the need to
.
- make risk assessment as objective as possible;
- make the results of risk assessments more understandable and
- establish more effective prioritization of limited, governmental resources


[In other words, the EPA should concentrate on problems other than second-hand tobacco smoke!
1992 Jan 28: Philip Morris's "Procurements & Payments" file for the Scientific Affairs (See last pages) lists Carlo as being paid $25,000. 

[This would be for Carlo's personal consultancy ('witness') work. The Bias Study and the Health Scientist Survey would have been paid separately]
1992 Feb: Consumers Research reported a speech by Vernon Houk where he claimed that the residents of Times Beach had been evacuated needlessly:
In summary, with the exception of chloracne... there are no convincing data for the association of dioxin exposure in humans, with early mortality, adverse reproductive outcomes, or chronic diseases of the liver or of the immune, cardiovascular, or neurologic systems.
The overall cancer question is not settled, but if dioxin is a human carcinogen, it is, in my view, a weak one that is associated only with high-dose exposures. [Consumer's Research magazine was run on the side by the same group who ran the National Journalism Center — and it was editorially available to any company which gave the NJC a healthy grant.]

1992 Feb: The Risk Analysis journal publishes Carlo's "Bias Study" under the title. "The Interplay of Science, Values and Experiences Among Scientist Asked to Evaluate the Hazards of Dioxin, Radon and ETS"
This study becomes widely cited by tobacco friendly scientists. But it is ignored by mainstream scientists, and seen as a thinly veiled attack on the European 'precautionary principle" — an approach generally applied only when it is difficult to accurately quantify the risk associated with certain actions/pollutants.
To investigate the extent to which personal values and experiences among scientists might affect their assessment of risks from dioxin, radon, and environmental tobacco smoke (ETS), we conducted an experiment through a telephone survey of 1461 epidemiologists, toxicologists, physicians, and general scientists. Each participant was read a vignette designed to reflect the mainstream scientific thinking on one of the three substances. For half of the participants (group A) the substance was named. For the other half (group B), the substance was not named but was identified only as Substance X, Y, or Z.
Knowing the name of the substance had little effect on the scientists' evaluation of dioxin, although those who knew the substance to be dioxin were more likely to rate the substance as a serious environmental health hazard (51% vs. 42%, p= 0.062).
For radon, those who knew the substance by name were significantly more likely to consider it an environmental health hazard than were those who knew it as substance Z (91% vs. 78%, p<0.001).
Participants who knew they were being asked about ETS rather than substance X were significantly more likely to consider the substance an environmental health hazard (88% vs. 66%, p<0.001), to consider the substance a serious environmental health hazard (70% vs. 33%, p<0.001), to believe that background exposure required public health intervention (85% vs. 41%,p<0.001), and to believe that above-background exposure required public health intervention (90% vs. 74%, p<0.001).
These findings suggest that values and experiences may be influencing health risk assessments for these substances, and indicate the need for more study of this phenomenon.

Note the discrepencies between these figures and those previously given to Philip Morris for publication in their book six months before (June 1991).
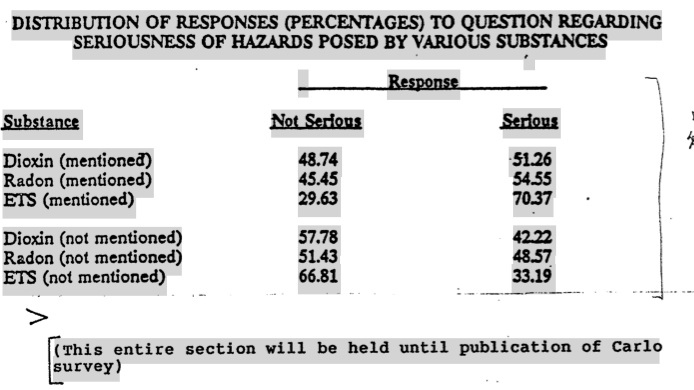
HES published different versions of the same study in different scientific and medical journals to get maximum exposure. This version is later quoted by Donna Staunton of the Australian Tobacco Institute in a submission to the National Health & Medical Research Council (NH&MRC) with the analysis that:
In so far as the evidence to be reviewed comprises epidemiologic data and statistical analysis of that data, it requires evaluation, inference and judgment. Thus, opinions as to the inferences etc. to be drawn from epidemiologic data differ from scientist to scientist.
Human nature being what it is, there is inevitably a degree of subjectivity involved and considerations of "social" or "political" correctness influence such deliberations.


1992 Feb 7: Philip Morris is circulating a memo about the "Health Science Survey on Risk Assessment." It is portrayed as proving that the public over-reacts to environmental health threats, and that Federal funds in the area of regulation and research are being misallocated. The executives are told that:
The attached survey of 1292 epidemiologists, toxicologists, clinicians and other health professionals was conducted by Dr George Carlo, chairman of the Health & Environmental Sciences Group (HES).[Note that they don't even admit to their own Corporate Affairs executives that this was a Philip Morris operation.
HES is a consulting firm located in Washington, D.C. which specializes in regulatory issues. The survey entitled "Identifying Consensus on Assessing Human Health Risk" was commissioned by the Institute for Regulatory Policy (IRP). IRP is a regulatory reform group lead by Thorne Auchter, former head of the Occupational Safety & Health Administration.
The Health Scientist Survey paper had still not been accepted for publication at this time.]
1992 Feb 7: The Wall Street Journal revealed that the Georgia-Pacific Company (a paper company later owned by Koch Industries) was forced to compensate two residents downstream of their mill $4.2 million over dioxin residues.
[The Koch Brothers through various family foundations were, and still are, the major funders of various libertarian think-tanks and policy institutes supporting the chemical industry over the dioxin war.]
Another 159 lawsuits has been filed against the company — which was not insured. Dioxins were also produced by incinerators and by cement kilns which had taken to using hazardous waste (mainly old tires) as a fuel,
[Also from another source which doesn't mention the Koch family]
Georgia-Pacific Co.— a major paper producer — recently lost two dioxin lawsuits in which juries awarded $4.2 million to residents living downstream of its paper mill on the Leaf River in New Augusta, Mississippi.
Georgia-Pacific has been named in 159 additional lawsuits filed by 8209 plaintiffs who claim they suffered emotional harm after eating fish contaminated with dioxins from the Georogia Pacific Plant, [and the] insurance carriers say their policites don't cover damages in lawsuits like these.
[ George Carlo had become a consultant to the trade association representing the cement kiln and incinerator industry.]

| Fall-out from the Banbury Conference |
|---|
1992 Feb 20: The Wall Street Journal published a front page expose of the Banbury Conference. This was followed a week later by an expanded review in Rachel's Hazardous Waste News (Mar 4) saying......[The WSJ] confirmed that the paper and chlorine industries have waged a successful two-year campaign to bamboozle the nation's media about the toxicity of dioxin, and that U.S. Environmental Protection (EPA) fell for it too.The Journal's story "Dueling Studies: How Two Industries Created a Fresh Spin on the Dioxin Debate" by Chicago-based staffer Jeff Bailey, describes a bald-faced campaign by the American Paper Institute (API) and the Chlorine Institute to "revisit" the scientific evidence that dioxin is a potent carcinogen. [Quoting the Journal] "The paper industry scored its first major public-relations success in 1990, when paper companies arranged to challenge the findings of the most influential dioxin study ever done. That study, reported in 1978 by Richard Kociba, a Dow Chemical Co. pathologist, was done on 485 white rats, whose food was spiked with dioxin. Dr Kociba found a strong link to cancer: a daily dose of billionths of a gram led to tumors."[Note Robert A Squire was another of Carlo's close associates. The uncertainty he noted probably reflects the experience and financial prospects of the participating ChemRisk scientists, — although biomedical scientists will squabble endlessly over whether a cell is 'cancerous' or 'pre-cancerous'. The article went on to discuss the way in which the campaign had developed, beginning in about the May 31, 1990,with Malcolm Gladwell's Washington Post article which surprised the world with the headline, ] "Scientists Temper Views on Cancer-Causing Potential of Dioxin."They commented that Gladwell's "chorus" consisted of quotations from four scientists and noted that he neglected to mention that three of them were consultants paid by the paper industry. With the Washington Post on board, the "de-toxify dioxin" campaign was rolling. "Next the Chlorine Institute... arranged to bring three dozen of the world's foremost experts on dioxin to a conference at the Banbury Center [on Long Island in October, 1990]." With the publication of the WSJ's story the "Dioxin Wars" campaign unraveled. The scientist in charge of EPA's reassessment, Peter Preuss, is quoted in the Journal saying that Vernon Houk's statements "misled" the public about the dangers of dioxin. Other scientists on EPA's reassessment team say dioxin seems to be just the tip of a nasty iceberg — that other chemicals in the environment seem to share dioxin's ability to interfere with the human reproductive and immune systems. If we all carry dioxin in our bodies at an average of 7 ppt [parts per trillion], when you add furans and PCBs [polychlorinated biphenyls], our average body burden of "dioxin equivalents" may be as high as 100 ppt. This is not good news. And it means that any additional dioxins or furans added to the environment would worsen a situation that it already unacceptable from a public health perspective. Rachel |
1992 Feb 22: Executive Order on Regulatory Risk Assessment:Jim Boland (Assistant to the CEO/Chairman of Philip Morris) to Craig Fuller (head of PM's domestic disinformation activities and ex-Chief of Staff in White House) who is in charge of getting the White House to issue an Executive Order to block the EPA from establishing its ETS Risk Assessment.
[Carlo's friend Thorne] Auchter believes that the Executive Order is on a fast track and that it will block EPA issuance of ETS risk assessment.
I am dubious since OMB [White House Office of Management & Budget] would be required to reject EPA's risk assessment without cover of previously delineated standards. Nevertheless, it is crucial that we resolve these questions ASAP, and finalize our plan.
Late Friday I learned that EPA may be embarking on a strategy to finalize the risk assessment before the Administration decides on actions resulting from 90 day moratorium/review — EPA may seek final risk assessment as earlv as April 1.
[A Presidential order had already blocked the EPA from issuing its Risk Assessment by creating a 90 day moritorium on its activities.]
As a result, I would suggest:I know it will be difficult by telephone, but it will give us the benefit of your thoughtt's — it is crucial that we finalize our plan by the end of'this week 2/28, if not sooner. (I am attachimg, another copy of the current proposed Executive Order for your convenience, and will fax materials which I expect from Auchter-Tozzi on Monday morning..
- It is premature to make copies of plan available (Auchter is even concerned about his name being on an ETS plan).
- It is important that you participate as much as you can in our discussion on Executive Order/Adtninistration policy on Monday, 2/24 at 1:00pm.


1992 Mar: Cantox, under the leadership of Dr Ian Munro prepares a very comprehensive report on likely contaminants of indoor air for the OSHA, US Department of Labor. They look at...
... 10 classes of biological agents, 230 chemicals, subdivided into 17 chemical classes, and 13 classes of physical agents.[There is no significant mention of tobacco smoke as a contaminant in this document. But note how Sick Building Syndrome disappeared from the public agenda almost immediately workplace smoking was banned.]
These agents are emitted by numerous sources which mainly comprise building materials and equipment (including furniture and HVAC systems), outdoor sources, and biological entities (e.g. humans and.plants). Sources related to normal (baseline) human activities (excluding smoking) also were identified and included mostly cleaning and renovation aetivities/materials.
Allergenic and pathogenic agents identified in the indoor environment are comprised mainly of fungi, virues and bacteria.. In line with this observation, a variety of bacterial and/or viral strains have been found to be responsible for the adverse health effects freqaently reported by building occupants.
Furthermore, the evidence available in the scientific literature associating HVAC systems, and more specificafly air conditioning systems, with SBS [Sick Building Syndrome] indicates that these biological agents, may represent a major cause.of complaints.
Major sources of emissions were identified as being mostly related to building materials (eg. renovations/painting) and equipment (eg. photocopiers)
Finally, in the case of SBS, there are actually no sound scientific criteria to understand, explain, or estimate the causes and effects related to: this event. Recent theories suggest that SBS may be considered the outcome of a multifactorial exposure comprising: physical, biological, and chemical as well as psychosocial factors. The interrelation between these components is still poorly understoodd and thus no predictive model is currently available.
The report does admit that
It. should be noted that the present report has purposely not discussed the contribution of environmental tobacco smoke to the indoor environment since this is an issue that has been extensively studied in numerous other reports. Instead, the report has concentrated on. the identification of potential health effects associated with sources of indoor air contaminants (IACs) in office environments other than tobacco smoke.
[In other words, Cantox prepared a report without discussing the contribution to ETS — the single most important factor — because it is a disputable issue (according to the tobacco companies).
They have a very strange view of 'science'.]


1992 Mar 13: Carlo's "The Health Scientist Survey (Identifying consensus on assessing human health risk)." is finally sent to the Environmental International journal under the IRP name.
1992 Apr: Carlo is on the list of consultants for Philip Morris for a fee of $15,000 per month.
This appears to be a retainer payment, rather than for any specific commissions — and it would have been for his media tours and witness statements (He was available for a set days a month).
The same document shows that his friends Jim Tozzi and Thorne Auchter are retained at $70,000 a month. 

1992 May:: The defence of the 2,4-D herbicide. George Carlo and Ian Munro, now seasoned firefighters in defence of dioxins, were trotted out once again to fight a growing firestorm over the less dangerous of this group of herbicides [Also a component in Agent Orange].
1992 Jul: This month's Environmental International journal carries the Carlo/Philip Morris "The Health Scientist Survey: Identifying Consensus on Assessing Human Health Risk" They had received the paper only on Mar 13 of that year. The abstract describes the methodology as:
The participants were asked questions concerning the relative usefulness and importance of specific types of data in defining health risk and were also asked to evaluate short vignettes describing various data profiles. Finally, participants were asked if they agreed or disagreed with a series of statements regarding public health policy, risk assessment, and risk managemen[:The also acknlowedge that
The survey was conducted during 1990 and 1991 with the support of the Institute for Regulatory Policy.Once again they forgot to credit Philip Morris.


1992 July 22: Executive Order on Regulatory Risk Assessment: Carlo's associate (later secret partner) Thorne Auchter is selected as a member of the President's Commission on Risk Assessment and Management at the White House which has been given some oversight over the EPA, FDA and OSHA regulatory standards and requirements (along with the OMB and OIRA). Another tobacco lobbyist and a lobbying lawyer for the nuclear waste industry are also selected.
The aim of this corporate-friendly commission is to lobby President Bush to sign an Executive Order requiring the regulatory agencies to all do both a formal 'Risk Analysis' and a cost-benefit study before making any new rules governing poisoning or polluting substances. After the hammering of the Reagan Administration, George Bush was reluctant to be seen as a friend of the polluters.
[These Executive Orders mandate that federal agencies must wait to impose rules until universally accepted estimates of the cause and effecta of the hazard to be regulated are known... a near-impossible task.
Regulators often know that a substance or product is dangerous long before they can measure the exact magnitude of the harm... or the full extent of the exposure... or even the exact mechanism by which a substance acts on the human body or environment and how that differs from their test animals.
In Europe, this public safety-first process was formalized as the "precautionary principle" but in the USA it was inverted in favor of the corporations.]
1992 July 31: Tom Borelli at Philip Morris is using a power-point presentation with material based on Carlo's Bias Study. [He also mentions Michael Gough as an 'independent' authority!],
Borelli tells his audience that the study's
Hypothesis: is that the use of the term ['ETS'] causes people to look at the science differently; [They believed] ETS was "bad" no matter what the science said.Essentially this is an attack on the EPA, claiming that it uses "junk-science" in its decision-making processes. He maintains that with ETS
[The trick is in Borelli's practiced use of scientific terms in imprecise ways. Seven of the 31 studies did find STATISTICALLY SIGNIFICANT risk associated with second-hand smoke.
- Available animal inhalation studies [were] ignored.
[How do you do a passive smoking study on mice? You'd need tens of thousands of mice housed in a smoke-filled casino to get even a few extra deaths in their 18 month lifespans.]- 31 Epidemiological Studies Considered,
- 24 Report No Statitically Increased Risk
- Yale University Study [The Varela Study]
- Largest Completed study [on passive smoking] in the US,
- [Which found] "No statistically Significant Increased Risk in Workplace, to Spouse at Home, Social Setting."
Most of the 24 studies which found no STATISTICALLY SIGNIFICANT increased risk, did, however find MEASURABLE increased risk and reported it as such.
[Independently-funded studies rarely enlist enough cases to show high levels of significant risk when dealing with widespread and complex pollution effects like second-hand smoke (which is only one component of polluted air). This is why the EPA and other regulators use statistical tools like meta-analysis to extract statitically significant data by combining a number of smaller studies.]


1992 Aug: /E Executive Order on Regulatory Risk Assessment: The July Monthly report of Government Affairs division of Philip Morris reveals that Thorne Auchter's Institute for Regulatory Policy (IRP) had also been behind another survey:
With regard to environmental tobacco issues, the Washington Relations Office continued its work with Corporate Affairs and PM USA to urge the Administration to issue an executive order that would establish procedures to enforce the application of sound scientific principles on all federal risk assessments.[This type of survey is pure motherhood stuff generated for the media to sensationalise.]
In support of those efforts, Market Strategies, on behalf of The Institute for Regulatory Policy, conducted a nationwide survey which demonstrated overwhelming public support for basing environmental regulations on sound science (88%) as well as public concern that if regulations are driven by media or political concerns rather than science, American taxpayers can be hurt by such regulations (83%).
Nevertheless, it appears that the EPA and the environmental groups have succeeded in blocking an executive order until after the election.
Although the Administration's deferral of an executive order was a significant setback, we will continue our aggressive efforts to require the application of sound science to the ETS risk assessment. Our focus must now be on the Energy and Commerce committeee's requested investigations.
It is essential that the scope of such investigations examine the bias which permeates the EPA's process on ETS, including the risk assessment.


The IRP had a monthly budget from Philip Morris around this time of $25,000
See supplementary budget explanation 

1992 Aug 3: THE tobacco lawyer-lobby firm, Shook Hardy & Bacon, have the full details of the Health Scientist Survey in their newsletter on passive smoking. 

1992 Aug 7: The Office of Criminal Investigations at the EPA announced tht it had closed its investigation into the Cate Jenkins charge that Monsanto had produced fraudulent studies on the safety of dioxins. They claimed that the time delay had stretched beyond the statute of limitations, and that the studies hadn't been relied on by the EPA anyway.
On July 20 1994 another internal EPA memo [William Sanjour to his supervisor David Bussard] detailed the charge that this inverstigation and the closure itself were fraudulent:
'One gets the impression, on reviewing the record, that as soon as the criminal investigation began, a whole bunch of wet blankets were thrown over it,' Sanjour told Bussard, noting that the investigation centred on criminal fraud.[Source: The Dioxin Wars by Robert Allen (Sunday Times, UK)]
'In August 1992, EPA quietly closed the criminal investigation without ever determining or even attempting to determine if the Monsanto studies were valid or invalid, let alone fraudulent.'
1992 Aug 12: John D Graham had asked Rick Guardia at Kraft General Foods (the Philip Morris food subsidiary) to help him raise $25 million for his Harvard Center for Risk Analysis.(HCRA)
The letter has also been ccd to the main tobacco staff, Tom Borelli, Mayada Logue (who had set up the initial contact with Graham), Bob McVicar who ran the KGF Foundation (which would launder payments as grants) and Jim Serafino, the head of "Scientific Affairs" [Issues management] for KGF. 

[Carlo is intmately involved in later times with Graham and the HCRA]
1992 Aug 21: Executive Order on Regulatory Risk Assessment: A memo to Betsy Annese, (the head of Government and Public Relations at RJ Reynolds Tobacco) by lobbyist Matt Swetonic. Swetonic is both introducing and promoting Carlo, who is seeking funding for a project to force through an Executive Order.
Until now, Carlo's tobacco work had been for the Tobacco Institute and Philip Morris, but now he is widening his catchment area to include RJ Reynolds.
Swetonic was famously the front-man for Johns Manville (the asbestos company) and also an external PR for Dow Chemicals (Agent Orange) working through E Bruce Harrison [EBH — at this time a subsidiary of Hill & Knowlton]. He wrote to Annese.
As we have already discussed, yesterday I met in Washington with George Carlo, head of the Health & Environmental Sciences Group, a DC-based health sciences consulting firm.Swetonic is advising Reynolds that Carlo would be the ideal man for this industry project.
I have known Carlo since 1983, when he was part of my "flying circus" of Dow scientists touring the country talking about the Agent Orange issue. As recently as three or four years ago, he had a working relationship with EBH doing joint projects for various chemical industry clients. In short, we know Carlo very well and, as a result, he was quite open and. candid in the meeting.
The [Carlo] study you sent me from Risk Analysis was funded through the Institute for Regulatory Policy which, as you know, has been the lead group trying to pressure the White House to release the Executive Order on risk assessment reform.
According to Carlo, EPA was so concerned about the implications of the study that he was called to a meeting at the agency to explain "what the hell I was up to."
Regarding the Risk Analysis study, since, as I relayed to you previously, Carlo is unwilling to speak out directly in support of ETS, his "handlers" at the "primary funding organization" [Philip Morris] did little to publicize it when it was published in March. Carlo believes, and I tend to agree, that it is probably too late to make much of a splash with it now. But there is something else he believes can be done.
Carlo was personally involved in a number of meetings at the White House on the Executive Order and he believes, as does Ward Hubbell, that the primary reason the Order was never issued was because it would have been perceived as a caving-in to industry at the expense of the public health.
[The Executive Order Swetonic speaks about is one that was designed to impose endless analytical requirements on government agencies before they could regulate on any potentially dangerous substance. Clearly, Carlo was now tied into some sort of partnership arrangement with Thorne Auchter at the IRP.
N Ward Hubble was a lobbyist-asscociate of Swetonic at EBH who fronted for RJ Reynolds via the National Environmental Development Association (NEDA) while Swetonic once ran the more widely tobacco-funded coalition known as the Total Indoor Environment Quality (TIEQ) organization. EBH, the NEDA and TIEQ all shared the same phone number. ]

According to Carlo, Boyden Gray "screamed" (his word not mine) at them at one meeting that they had blown the whole thing by their heavy-handed corporate lobbying tactics and that he couldn't afford to hand the Democrats one more issue to beat President Bush over the head with in an election year.
[ C Boyden Gray was an heir of the RJ Reynolds tobacco fortune, and also White House counsel to Presidents Reagan and Bush During the Clinton Administration he ran the think-tank Citizens for a Sound Economy (now FreedomWorks) which supported the tobacco industry in exchange for generous funding, and since its inception, he also chaired the Executive Board of both the Harvard Center for Risk Analysis]
Carlo's idea for resurrecting the Executive Order is to create a groundswell of support among scientists for massive changes in the way regulatory agencies assess risk. He believes this would enable the White House to issue the Order, not because industry wants it, but because the scientific community believes it to be necessary.
- The EPA = junk-science line was an extension of Philip Morris's funding of The Advancement of Sound Science Coalition (TASSC) which was in the process of being created under Borelli and Ellen Merlo.
- Enlisting pedantic scientists to insist on 'sound-science' became a key part of the tobacco industry's "Heidelberg Appeal' attack on the 1992 Rio Earth Summit.
- Adding risk analysis before regulating, became part of the TASSC project supported by PM and Reynolds via Steve Milloy's junkscience.com web site.
- The Executive Order they were proposing attempted to strangle agency regulations by imposing impossible conditions on them — specifically Risk Assessment and cost benefit analysis. Both put economists in the box-seat of regulatory decisions, and devaluated the expertise of bio-medical and environmental scientists.
- The Executive Order they eventually got from President Clinton was less than satisfactory in the industry's eyes (but still a burden on the agencies) ].


Swetonic characterised Carlo's Health Scientist Survey and Bias Study as:
There were actually two studies published as part of the IRP effort to demonstrate to the White House that the scientific community had little faith in the way regulatory agencies were assessing risk.
The second, a copy of which is enclosed, asked a ton of questions to scientists regarding what types of evidence they would consider important in assessing risk. A number of the questions relate directly to the ETS situation, although none address it by name.
The second study, in particular, received some favorable publicity in the environmental trade press around the beginning of this year.
perceived the relative risks of dioxin, radon and second hand tobacco smoke. The conclusion was that the public perception was that all three risks were of comparable concern |
1992 Sept: The International Journal of Toxicology published "Comprehensive, Integrated Review and Evaluation of the Scientific Evidence Relating to the Safety of the Herbicide 2,4-D" by George Carlo, Ian Munro and the joint HES and Cantox staff. The two companies are now working closely together. 
1992 Sep 25: The Times reported that Dr Vernon Houk from the US Public Health Service, had been criticised by Congress, the National Academy of Science, and others.
He was the "unnamed federal official" who had ordered the dioxin-related evacuation of Times Beach, Mo., and who later maintained the company-inspired line that the evacuation was unnecessary.
[Houk] admitted copying virtually verbatim from Dow Chemical documents in proposing relaxed standards for dioxin.A couple of years before, a number of top EPA officials had been forced to resign (seven in all) because of their close associations with various corporations. One of these officials, John Hernandez, had also been taking his written regulatory material straight from Dow Chemicals documents.
{Houk appears to be more gullible than corruptable. He trusted corporate scientists to be honest, and he fought for good causes. He was instrumental in the battle to remove lead from paints and gasoline, battled the US government over exposures to atomic bomb testing — yet he also became a major figure on the chemical industry side over dioxins and Agent Orange. He died of cancer in 1994.]
1992 Dec: Kelly Sund is George Carlo's stand-in on the agenda of speakers for the Risk Communications Specialty Group, at the Annual Conference of the Society of Risk Analysis held in San Diego. 

1993-95: Carlo began to create a myriad of nonprofits and pseudo-science panels and societies in the early-to-mid 1990s. We have no exact foundation dates for most of these.
1993: The Chlorine Institute on behalf of the chemical industry began a $5 million public relations campaign with the release of a report entitled "Scientific Principles for Evaluating the Potential for Adverse Effects from Chlorinated Organic Chemicals in the Environment."
This new report, was written by Carlo's business associate Ian Munro via his Canadian consulting firm CanTox, and it argues that concerns about organochlorines are overstated, and must be addressed within the current regulatory framework — one chemical at a time (there are hundreds of dioxin-producing chemical processes).
1993: The Nufarm dioxin spill: Nufarm [The Australian company with the 1990 dioxin problem in Melbourne — see Part 1] was a subsidiary of Fernz Pty Ltd. a New Zealand company [itself a subsidiary of Dow] which also owns Pharma Pacific and Pharma Pacific Management Pty Ltd. George Carlo was listed as Technical Director for all these companies and Carlo was sent on media and scientific conference tours by Pharma Pacific.
An approach made in 1998 to Pharma Pacific Management for them to provide a "dioxin expert to present at a risk conference" revealed that Dr Carlo was still being offered around the world at this stage as a keynote conference speaker by the Pharma Group (They paid the airfare but not the lecture fee). He was then being touted by Pharma as an expert on 'Risk Assessment'. [They didn't say who's risk!
In 2010, Pharma Pacific and NuFarm Ltd were now both listed at 103-105 Pipe Road Laverton, Victoria in Australia.
In this year Pharma Pacific also incorporated in Phoenix, AZ using vague terms like "Manufacturing Services"]
1993: The Nufarm dioxin spill: (Following the Nufarm dioxin spill in Melbourne water supply) the Australian Journal of Public Health publishes: Background soil concentrations of phenolic compounds, chlorinated herbicides, PCDDs and PCDFs in the Melbourne metropolitan area. by George Carlo, Kelly Sund, RL Crouch & BC Senefelder.
1993 Jan: /E Executive Order on Regulatory Risk Assessment: Tom Borelli's report on "New Project" for Philip Morris lists:
While the ETS budget was originally set-up to address only that issue, part of the funds went to establish groups (IRP) and resources (EPA Watch) that have a broader impact for PM.
All of these consultants have been very effective in addressing the ETS issue for PM and could assist PM in other regulatory matters. Tozzi/Auchter, have excellent government contacts which makes them a valuable resource for the Washington office.
[Carlo also worked with them on the Executive Order]

Auchter — Last year, PM contributed [$] 880k (600k from Steve [Parrish], 280k from Craig [Fuller]) to establish the Institute for Regulatory Policy (IRP) as a vehicle for the executive order on risk assessment. Although the executive order did not make it, IRP is now a viable organization that can address various regulatory issues.
IRP has established a coalition representing the interests of a broad array of industries and trade associations. Additionally, IRP has established a relationship with many state and local governments throughout the US. The coalition could address a number of regulatory issues of interest to PM in 1993.
Since we have already made a sizable investment in establishing IRP, it would make sense to keep it going (at some level) for future issues.


Cellular Telephone Industry Associations' (CTIA) Scientific Advisory Group.
[He was, of course, completely devoid of any knowledge of bio-electro-magnetic research,
EMFs, Radio-Frequency, microwaves, exposure systems or cellular phone technologies.]
See Part 3 — Cellphone EMF problems: the CTIA and SAG/WTR
1993 Apr 12: Jim Tozzi and Thorne Auchter (possibly with George Carlo) established a Center for Epidemiological Studies as a subsection of their highly profitable/non-profit Federal Focus. Inc which was their major health-related lobbyshop. Tozzi wrote to Philip Morris offering services:
As we previously discussed, EPA is placing an increasingly greater emphasis on epidemiological studies as a basis for its risk assessment and rulemakings. To address this need, we have established the Center for Epidemiological Studies as part of Federal Focus, a 501(c)(3) corporation.This was likely created to push the "Good Epidemiological Practices" (GEP) standards. Philip Morris spent a small fortune on lawyers used to write up model legislation for the European Parliament, and then ran a number of symposia in parts of Europe. They were probably organised through this pseudo-organization.
The Center is staffed by several fulitime epidemiologists who are supported by a number of internationally known epidemiologists on a contractual basis. The Center staff will play an increasingly greater role in the ETS risk assessment.
The nominal "Center Director" was Gary Kayajanian, PhD. and its major effort in its first years appears to have been data-mining the citations on ETS research to discover any which could be used against the Environmental Protection Agency.


1993 Apr 22: With the Washington Legal Foundation (WLF) [A corporate funded anti-regulatory think-tank and legal advice center] George Carlo and James Baller jointly write:
- "Counsel Advisory" for: "Business Exposed To New Liability With Upcoming Health Assessments of Superfund Sites" which...
...provides a timely, provocative alert to the business community of the need to comment on the Agency for Toxic Substances and Disease Registry's pending health assessments of waste sites.


- Publish with Barry L Johnson, Assistant US Surgeon General and Assistant Administrator of the Agency for Toxic Substances and Disease Registry (ATSDR) "What You Need to Know About the ATSDR's Role In the Superfund Decision-Making Process."


- Run a luncheon seminar with Johnson. this year (date uncertain) through the Washington Legal Foundation on "How to Minimize Superfund Liability by Successfully Handling the ATSDR Health Assessment Process."
1993 May: (On the EPA classifications of dioxins.) Mark Shannon, (associate director in Washington of Ketchum Public Relations) began for Dow Chemicals...
... a blitz, which started in May and covered at least 30 cities, [and] was designed to undercut the press coverage that the EPA report was expected to receive when it was released in September.[This must have been an extension of the famous "flying circus" refered to by Matt Swetonic in a memo to RJ Reynolds]
"Basically what we're trying to do is assure that industry's voice is heard by people who make policy decisions both here and around the country," Shannon said.
The CMA is also providing about $200,000 to finance a scientific review panel to provide comments to EPA about the quality of its dioxin reassessment. The panel is co-chaired by John A. Moore, an EPA acting deputy administrator during the Reagan Administration who is now the president of the Institute for Evaluating Health Risks, a Washington think tank.


1993 June: The Nufarm dioxin spill: Two of Carlo's 1990 Melbourne water-quality/dioxin papers were published in the Australian Journal of Public Health. Both listed as research associates, Kelly Sund and James Baller.
- Assessment of dioxin-related health risks for the Melbourne metropolitan area [See listing in 1990]
- Sund appears to have no biomedical degree and worked for him at HES as his private secretary and general factorum.
- James Baller was his contract lawyer who later did work for him on a cellphone radiation panel when he ran the cellphone operation (WTR).
These three "independent" experts found no cause for the Melbourne citizen's alarm, and Carlo told the Australian media that adverse health effects are unlikely to result from general population exposures to PCDDs and PCDFs. This was reported in the newspapers as clearing the Melbourne Water Supply of any suspicion of contamination.
At this time Nufarm was a subsidiary of Fernz Pty Ltd. a New Zealand company which owns Pharma Pacific and Pharma Pacific Management Pty Ltd. George Carlo was, at this time, listed as Technical Director for all these companies and Carlo was sent on media and scientific conference tours by Pharma Pacific.
[An approach made in 1998 to Pharma Pacific Management for them to provide a "dioxin expert to present at a risk conference" revealed that Dr Carlo was still being offered by them as a keynote conference speaker.
He was then being touted by Pharma as an expert on 'Risk Assessment'. They didn't say who's risk! Pharma Pacific and NuFarm Ltd are now both listed at 103-105 Pipe Road Laverton, Victoria in Australia. In 2010 Pharma Pacific also incorporated in Phoenix, AZ using vague terms like "Manufacturing Services"]
"Background soil concentrations of phenolic compounds, chlorinated herbicides, PCDDs and PCDFs in the Melbourne metropolitan area" by Kelly G. Sund, George L. Carlo, Richard L. Crouch and Brian C. Senefelder
Abstract: During June and July 1990, surface soil samples were taken in the Melbourne metropolitan area and analysed for phenolic compounds, chlorinated herbicides, polychlorinated dibenzo-para-dioxins (PCDDs) and polychlorinated furans (PCDFs). A surface soil sample from a Werribee Farm Treatment Complex paddock, where cattle graze on land that is used for filtration of sewage, was also taken and analysed.
No phenolic compounds or chlorinated herbicides were detected at the parts per billion detection limits in any of the samples. PCDDs and PCDFs were detected in both industrial and nonindustrial sectors of the Melbourne metropolitan area, as well as in effluent from Nufarm Limited, an agricultural chemicals manufacturer in Laverton North, in similar concentrations (toxic equivalents in the parts per trillion range). These concentrations were consistent with background levels identified in other major urban areas. There was no evidence of the Nufarm effluent fingerprint in any of the background soil samples analysed. The fingerprint profile of the sample taken from Werribee Farm, although showing traces of the Nufarm effluent fingerprint, was clearly distinct from that effluent fingerprint and consistent with the fingerprint identified in the background soils.
The impact of the Nufarm effluent on the area, therefore, was considered insignificant.

1993 June: Carlo study in the Journal of Occupational Medicine "Reduced mortality among workers at a rubber plant."
[Larry Newman of NPL was PR for Bridgestone Tires]
We conducted a retrospective cohort study of mortality among current and former male employees of a modern tire manufacturing plant in Texas. Vital status was ascertained for more than 99% of the cohort of 2306 workers. Standardized mortality ratio analyses were completed based on 102 deaths. No significant excess for any disease-specific cause of death was identified, and significant deficits in all-cause mortality and deficits in cancer mortality were observed for both white and nonwhite men. The analyses were consistent in indicating that this work force has experienced no excessive disease-specific mortality

1993 June 8: "Evaluation of the Origin, Emissions and Controls of Organic and Metal Compounds from Cement Kilns Co-fired with Hazardous Wastes." A report by the "Process Technology Workgroup" of the industry (Cement Kiln Recycling Coalition) which has been sent to the EPA.
This report has been peer reviewed and approved by the' following members of the Scientific Advisory Board on Cement Kiln Recycling:Technical assistance was provided by
- George L. Carlo, Ph.D., M.S., J.D., SAB Chairman
(Health & Environmental Sciences Group, Ltd., Washington, D.C.)- Russell Keenan, Ph.D.,
(ChemRisk, Inc., Portland, Maine)- Harold Imbus, M.D., Sc.D.,
(Health and Hygiene, Inc., Greensboro, North Carolina)
- Drs. Michael P.Heap and Warren D. Owens, Reaction Engineering International, Salt Lake City, Utah,
- Maureen Jablinske, Health & Environmental Sciences Group, Ltd., Washington, D.C., and
- James Baller, Esq., Baller Harnmett, Washington, D.C., Special Counsel to the SAB.
 [Russel J Keenan was a member of Dow's Kociba-study re-evaluation group along with Dennis J Paustenbach. The rest were Carlo staff or associates.]
[Russel J Keenan was a member of Dow's Kociba-study re-evaluation group along with Dennis J Paustenbach. The rest were Carlo staff or associates.]
1993 July 9: Science magazine reported that Carlo's associate, Ian Munro of Cantox had also been employed by the Chlorine Institute. Cantox had produced reports which argue that the dangers of chlorine compounds (ie dioxins) have been exaggerated.
1993 July 12 - 16: The Toxicology Forum is held in Aspen, Colorado. Speakers on Herbicide problems include:
- Evaluation of epidemiology studies and perspectives on weight of evidence — George L. Carlo,
- Exposure and toxicity of 2,4-D — Ian C. Munro, CanTox, Canada


| Washington Legal Foundation (WLF) |
|---|
| "Free enterprise advocates with public interest know-how". It's mission: "promoting free enterprise principles; limited government property rights; and reform of the civil and criminal justice system." [and] "shaping public policy through aggressive litigation and advocacy". [Source: old WLF website] |
1993 Aug 6: The Washington Legal Foundation published a couple of Carlo 'Legal Backgrounder' papers on Environmental Regulation. The joint authors are:
- Dr Barry L. Johnson. Assistant U.S. Surgeon General and Assistant Administrator of ATSDR [Agency for Toxic Substances and Disease Registry],
- James Baller, a senior principal of the Washington, DC law firm of Baller Hammett, P.C., [Carlo's friend and associate on Melbourne dioxin study]
- Dr George L. Carlo, an epidemiologist and Chairman of the Washington, D.C.-based Health and Environmental Sciences Group, Ltd.
What You Need To Know About ATSDR'S Role In The Superfund Decision-Making Process (4 pages)...[which] discusses the expanding importance of the ATSDR in the Superfund decision making process.
The paper also urges greater involvement by industry with ATSDR could create important new opportunities to influence EPA's decisions for those who can understand ATSDR's procedures and multi-disciplinary thought processes and participate effectively in the health-assessment process on a site-by-site basis.
 On Apr 22 they also published another WLF paper on "Business Exposed To New Liability With UpComing Health Assessment of Superfund Sites" which
On Apr 22 they also published another WLF paper on "Business Exposed To New Liability With UpComing Health Assessment of Superfund Sites" which Provides a timely, provocative alert to the business community of the need to comment on the ATSDR's pending health assessment of waste sites.And they also provided a commentary to the Congressional hearing on the same subject.


Another similar paper by the same authors is listed in the WLF books as: "How to Minimize Superfund Liability by Successfully Handling the ATSDR Health Assessment Process."


1993 Nov: TASSC: The Clinton Administration was planning to raise the Environmental Protection Agency (EPA) to cabinet-level status. This scared the hell out of the big corporations and the tobacco industry.
They needed a way to counter the EPA's claim to scientific rigor, so Philip Morris, via their private PR firm, APCO, funded a supposed 'grassroots watchdog" organisation called The Advancement for Sound Science Coalition (TASSC) which had only one purpose; to attack the 'junk-science' used by regulators such as the EPA. It purported to be a grass-roots organization of concerned scientists.
They hired ex New Mexico Governor Garrey Curruthers to be President and founder, and to conduct media tours promoting the tobacco industry's sound-science/junk-science message. The [then unknown] Steve Milloy acted as the behind-the-scenes media contract for TASSC and then became its director after Curruthers retired.
With a lot of expensive promotion, Curruthers early media tours were highly successful (see two of many examples of news stories).
- Nov 28 1993 Denver Post published their 'scoop': "Here's how science might be used to keep science honest.
The stated mission of the nonprofit corporation is to use its own scientific panel to "inform public officials, the media, and the general public about the consequences of inappropriate science," focusing on current examples of "unsound government research used to guide policy decisions." Compliance with environmental regulations cost the nation an incredible $115 billion in 1991 alone"
- Jan 2 1994 The Tampa Tribune published Slogans no substitute for sound science. [Syndicted from LA Daily News]
Among TASSC's goals: to Inform the general public about the consequences of inappropriate science by focusing attention of current examples of unsound government research used to guide policy decisions; to establish an educational out-reach program; and to offer resources to ensure that sound scientific principles are applied.
The LA Daily News columnist Michelle Malkin who used her columns to attack Greenpeace and other activists, either through gullibility or self-interest, suggested that readers sign up for a free membership to TASSC.
Unfortunately, such skeptics as Bruce Ames, Lois Gold, Dixy Lee Ray and the late Aaron Wildavsky don't get attention with the same ease as star-studded environmental groups and enterprises like Greenpeace and the Walden Project.
[Obviously, from her numerous later favourable columns on the subject, 'Mickey Malkin' became a card-carrying TASSC member herself! The following year she was also working on favourable tobacco stories with the Competitive Enterprise Institute (CEI) and publishing pro-tobacco and anti-EPA op-eds in the Washington Times.]
- seemingly gullible scientists who thought membership of such a non-profit might earn them speaking engagements at Florida conferences during winter.
- PR/lobbying executives from some of the major polluting companies.
- businessmen who didn't like environmentalism, and believed most of the climate and environmental science was concocted propaganda,
- numerous academics and scientists who worked indiscriminately for the polluting industries, provided they were well compenstated


1993 Dec 21: Tom Hockaday and Katie Dold of APCO reported to Philip Morris about the "International Press Briefing on The Advancement of Sound Science Coalition (TASSC) which had had acceptances from 24 journalist.
APCO and Philip Morris were in the early stages of planning ways to extend the role of TASSC, and so gain control of the 'sound science' debate in Europe.
In the USA, TASSC had been successful beyond their wildest dreams. It had begun leading attacks on the 'junk-science" it said was behind the regulation of second-hand smoke, cellular telephone and EMF concerns, nuclear waste disposal, food processing, and global warming. TASSC had quickly been accepted by the media as the 'scientific authority' in labeling good and bad science, simply by claiming to be a grassroots society of 'concerned scientists.
The exclusive funding of Philip Morris was now being replaced and expanded by grants from other companies and other industries; the 'junk-science' message had almost universal appeal among corporate poisoners and polluters.
A questionairre sent to prospective members asking them to indicate their "junk-science" concerns, is quite revealing since it illustrates the primary concerns of the funding organizations:
[Carlo would have been involved in providing science-services on about half of these junk-science concerns. So it is not surprising to find him on the Scientific Advisory Board of TASSC.]
TASSC itself was a copy of Elizabeth Whelan's American Council for Science & Health (ACSH) which had successfully established itself (with chemical industry funding) as the arbiter of what was good and bad regulatory science for pesticide residues, food additives/preservatives, etc..
ACSH had scored over the health-activists by running a campaign to ridicule their exaggerated claims about the danger of Alar on apples. This counter-measure appealed to conservatives in both of the main political parties, and quickly won Whelan the support of pesticides and food additive companies. [She had previously worked for the Chemical Manufacturer's Association (CMA) and its American Industrial Health Council (AIHC) which lobby for the companies in the area of health and the environment science. Her partner, Frederick Stare worked mainly for the food processing industry, and helped tobacco on the side (Carl Seltzer).]
However, the tobacco industry couldn't use ACSH because Whelan had gained her 'public-health-advocate' notoriety by attacking smoking and the tobacco industry as the principle cause of cancers (partly exonerating occupational chemical exposures). This forced Philip Morris to create its own 'junk-science' astroturf.
[Note that George Carlo was enlisted as a member of the TASSC's Science Advisory Board, but he was not on the Science Advisory Board of ACSH. Most of the other science-for-sale entrepreneurs tended to sign up to support both operations.]
Katie Dold (ex APCO) became administrator of TASSC, and when Curruthers left Steve Milloy shifted out from the back-room and proclaimed himself the newly elected "Executive Director". At about the same time they created the "junkscience.com" web pages — and Murdoch's Fox News gave Milloy a regular TV spot.
Milloy also ran the "Issues Watch" crisis monitoring service for the tobacco industry. The control of the whole operation later passed from Philip Morris to RJ Reynolds when documents were leaked exposing the role of PM in the organization and funding of TASSC.
CONTRIBUTORS:LR13 JJHS hrh2


This work is licensed under a Creative Commons Attribution 2.0 Generic License